Dr. Steve Lamberg says OSA is more than just AHI, and that clinicians should evaluate the severity of the disease process and the activity level that may quantify the risk of progression. Read more about a useful way to screen and stage OSA.
 by Steve Lamberg DDS, D.ABDSM
by Steve Lamberg DDS, D.ABDSM
Julie has an AHI of 32. Her apnea is far worse than Steve’s because his AHI is only 12, right? What if Steve had events lasting 47 seconds and O2 desaturations to 63%? Does his disease still seem mild to you? Does Julie’s apnea seem as severe in comparison?
For more than 50 years the existence and severity of sleep related breathing disorders (SRBD) such as obstructive sleep apnea (OSA) have been quantified using the apnea hypopnea index (AHI). The AHI is based on data derived from an overnight sleep test such as a polysomnograph (PSG) or home sleep test (HST). SRBDs are conditions of abnormal and disordered breathing during sleep and include a spectrum of airway problems such as snoring, upper airway resistance syndrome (UARS), mild/moderate/severe OSA, central apnea, and hypoventilation syndrome.1
Because OSA presents with variable amounts of oxygen deprivation and sleep interruption, these scored events do not always correlate with levels of disease. Some patients with very few scored airway events may have more significant disease than others who have a higher number of “scored” events.2 This disparity between the number of events and levels of disease necessitates more revealing metrics beyond the constraints of AHI, so clinicians can more accurately assess the actual disease level and its risk of progression for an individual.
In recognizing the broad spectrum of clinical and pathophysiological features that OSA covers we may be able to take advantage of this opportunity to promote precision medicine and focus on various treatable traits that constitute this heterogeneous disease. Looking through this new lens also offers a more meaningful approach to evaluating the efficacy of treatment(s).
Other reasons to transcend AHI include the following: the lack of standard definitions for hypopneas and apneas, competency gaps of scorers create inconsistencies, missing information which includes the duration, depth, and distribution of oxygen desaturations, and an appreciation that variable patient phenotypes may lead to disparate consequences.
The best-practice in sleep medicine requires that we evaluate both the severity of the disease process as well as the disease activity level which quantifies the risk of progression. Our new understanding of the connections between SRBDs and overall health and wellness leads us to appreciate the advantages of recalibrating our diagnostic approach to include the “Staging and Grading” of SRBDs.
Staging is a way to classify the severity and extent of the systemic effects to an individual based on the measurable extent of destroyed tissue and or damaged systems which are attributable to airway problems. It is a measurement of how sick the patient is currently. Staging also assesses the complexity of controlling current disease as well as management of long-term consequences.
Grading is utilized to classify the risk of future progression of the airway disease and is based on disease activity levels in combination with the individual risk factors of the individual. Grading helps estimate the potential impact of airway problems on the individuals’ systemic disease going forward. Data collection to score grading is introduced below.
The proposed novel system advocates for the diagnosis of SRBDs to be reported as a stage and grade. As an example, a patient who has been previously diagnosed with severe sleep apnea in the presence of multiple comorbidities, could now be diagnosed as Stage 3 or 4 OSA; Grade A, B, or C depending on the risk of progression of the disease. This diagnostic system is superior at flagging a patient with a barely detectable airway disorder but a high disease level, such as in UARS, and exposes the need for treatment. Staging and grading will help us identify our patients’ disease profiles more fully and inform us of the appropriate treatment approach and its efficacy.
Staging and Grading of SRBDs
Staging: Disease “staging” criteria address which system(s) of the body have problems, the etiology or cause of the problems, and the pathophysiologic changes that have already occurred conferring a severity level or a degree of risk of disease complications. Data is collected from a patient’s history, the Lamberg Questionnaire v14, physical examination, and laboratory findings in order to more definitively diagnose the problem, prescribe appropriate treatment, and ultimately estimate the patient’s prognosis. Additionally, data indicating the presence of morbidity biomarkers, endothelial dysfunction, hormonal deficiencies, and negative response to treatments would factor in.
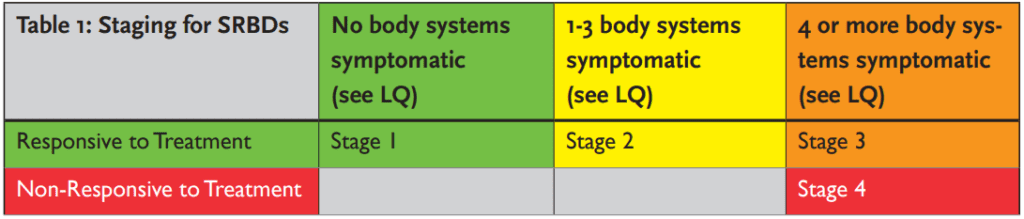
The Lamberg Questionnaire v14 can be used for screening and staging.3 It is segmented into medical categories, each representing a body system. Each medical category that has at least one symptom checked within it indicates the involvement of a body system affected by the SRBD. The recommendation for stage designations are as follows (see table 1).
Grading: The purpose of grading is to establish quality-assured screening for people with SRBDs based on activity levels of the disease. Grading is based on evidence of current disease activity and the rate of disease progression conferring a risk for the future.
Activity of the disease can be based on subjective reporting, changes in recent medical history, and the presence of systemic inflammatory biomarkers. An example of systemic inflammatory biomarkers that could be used to evaluate the disease activity include the following: hs-CRP, fibrinogen, erythrocyte sedimentation rate (ESR), uric acid (UA), TNF α, IL-6, IL-8, ICAM, and VCAM.4 The recommendations for grade designations are as follows.
- Grade A slow or no progression
- Grade B moderate progression
- Grade C rapid progression
Risk factors, or grade modifiers, may include anatomic features, physiologic profiles, behavioral problems, or preexisting medical conditions. For example, BMI, craniofacial profiles, smoking, diabetes, and decreased amount of slow wave sleep would contribute to the risk assessment of disease progression. Additional physiologic risk factors include: high Pcrit, low arousal threshold, high loop gain, and poor muscle recruitment. Data collection for these features will aid in the risk assessment of disease activity.
Discussion
The current diagnostic protocol for diagnosing SRBDs solely using AHI does not take into account the impact of the disorder on the individual patient. For example, patients with UARS may have a higher stage and grade designation than patients who have mild, moderate, or severe OSA, and sadly these patients are frequently overlooked due to the incorrect assumption by insurance companies that their low AHI confers a decreased medical necessity for treatment. It should be accepted that because all patients have a unique profile of medical risk to their body’s systems, based on genetic and epigenetic factors, staging and grading will establish a more complete picture of the SRBD and could even aid clinicians in establishing the most appropriate treatment choice(s) while also defining the efficacy of therapy.5,6 Evaluating success of treatment should include parameters such as quality of life and health outcomes as much as multiple objective metrics.7
Staging and grading classifications will evolve and become more useful to clinicians as our knowledge of the relationship between SRBDs and systemic disease is expanded.
Glennine Varga shows how to look for more than just AHI in children with OSA. Read her article, “AHI 1 All Night” here:
https://dentalsleeppractice.com/ahi-1-night/
- Guilleminault, C., Stoohs, R., Clerk, A., Cetel, M., Maistros, P., A cause of excessive daytime sleepiness. The upper airway resistance syndrome. Chest 1993 104(3), 781-787.
- Bouloukaki I, Grote L, McNicholas WT, et al. Mild obstructive sleep apnea increases hypertension risk, challenging traditional severity classification. J Clin Sleep Med. 2020;16(6):889–898.
- Lamberg, S., DDS. (2021). Lamberg Questionnaire Version 14. Retrieved March 30, 2021, from https://drlamberg.com/storage/app/media/seminars/lq-14-final.pdf
- Izolde Bouloukaki, Charalampos Mermigkis, Nikolaos Tzanakis, Eleftherios Kallergis, Violeta Moniaki, Eleni Mauroudi, and Sophia E. Schiza, Evaluation of Inflammatory Markers in a Large Sample of Obstructive Sleep Apnea Patients without Comorbidities, Mediators Inflammation. 2017; 4573756. Published online 2017 Jul 31
- Pevernagie DA, Gnidovec-Strazisar B, Grote L et al. On the rise and fall of the apnea-hypopnea index: A historical review and critical appraisal. J Sleep Res 2020;29(4):1-20.
- Won CHJ When will we ditch the AHI? J Clin Sleep Med 2020;16(7):1001-03.
- Sutherland K, Vanderveken OM, Tsuda H et al. Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med 2014;10(2):215-27.
 Dr. Steve Lamberg has been practicing comprehensive restorative dentistry in Northport, NY for 40 years. Always passionate about sleep and wellness, he became a Diplomate of the American Board of Dental Sleep Medicine in 2011 and has served on their board review faculty. He holds several patents, and is the inventor of the Lamberg SleepWell Appliance, which is FDA-cleared for the treatment of OSA. Dr. Lamberg also launched and serves as the director of the Pediatric and Adult Airway Network of New York (PAANNY), to provide a local platform where dentists, physicians, orofacial myologists, and other related professionals learn and collaborate on treatment patients of all ages. Additionally, Dr. Lamberg serves as a Scientific Advisor at the Kois Center in Seattle. His recently published book for the general public, “Treat the Cause…Treat the Airway” correlates many common medical conditions to airway and sleep and is available on Amazon.
Dr. Steve Lamberg has been practicing comprehensive restorative dentistry in Northport, NY for 40 years. Always passionate about sleep and wellness, he became a Diplomate of the American Board of Dental Sleep Medicine in 2011 and has served on their board review faculty. He holds several patents, and is the inventor of the Lamberg SleepWell Appliance, which is FDA-cleared for the treatment of OSA. Dr. Lamberg also launched and serves as the director of the Pediatric and Adult Airway Network of New York (PAANNY), to provide a local platform where dentists, physicians, orofacial myologists, and other related professionals learn and collaborate on treatment patients of all ages. Additionally, Dr. Lamberg serves as a Scientific Advisor at the Kois Center in Seattle. His recently published book for the general public, “Treat the Cause…Treat the Airway” correlates many common medical conditions to airway and sleep and is available on Amazon.