1. How do I earn Continuing Education (CE) credits through the webinar?
Answer: To earn one CE credit, attend the live webinar at the specified date and time using the provided Zoom join link. Afterward, successfully complete a ten-question quiz on our publication’s online system.
2. How can I attend the live webinar?
Answer: Simply use the Zoom join link sent via email on the specified date and time. Ensure an internet connection and, for optimal viewing, use a laptop or desktop computer. If using a mobile device, we recommend the Zoom app for a smoother experience.
3. Can I watch the webinar at a later time?
Answer: Yes, registrants receive a replay link via email within one hour of the live event. This link, hosted on our publication’s website, allows you to watch the replay at your convenience.
4. How can I ask questions during the webinar?
Answer: Use the question box in the webinar system to type your questions or comments. The moderator encourages attendees to do so multiple times before and after the presentation. Questions are addressed at the end of the presentation.
5. What if my question isn’t answered during the webinar?
Answer: If a question isn’t addressed live, we forward it, along with your email address, to the presenter for a direct follow-up.
6. How do I access the CE quiz?
Answer: Sign in if you’re a previous webinar attendee or sign up if you’re a first-time attendee on our publication’s website. The ten-question quiz is linked on the replay page and available directly through the CE dashboard after logging in.
7. How do I obtain the CE certificate?
Answer: After successfully completing the quiz, you can download a PDF of the certificate by clicking the provided button. Additionally, a copy of the CE certificate is emailed to the address on file.
8. What if I face issues accessing webinar-related features?
Answer: Reach out to us via email, chat, or phone for assistance. We can guide you with simple steps and screenshots on navigating and completing the webinar CE quizzes.
9. How long is the webinar valid?
Answer: The webinar expires two years from the live date. Ensure completion of the CE quiz within this timeframe to receive credits.
Please note:
While we take reasonable care in providing accurate content, opinions expressed in the materials are those of the presenter, not the CE provider. These materials are intended to supplement, not substitute, the knowledge of healthcare professionals.
10. What should I do to ensure email deliverability for CE credits?
Answer: Check your spam/junk folder for emails from subscriptions@endopracticeus.com and set this address as a safe sender in your email platform.
11. How can non-subscribers set up an account for the CE quiz?
Answer: Non-subscribers can set up a free account on our publication’s website to access the CE quiz and earn credits.
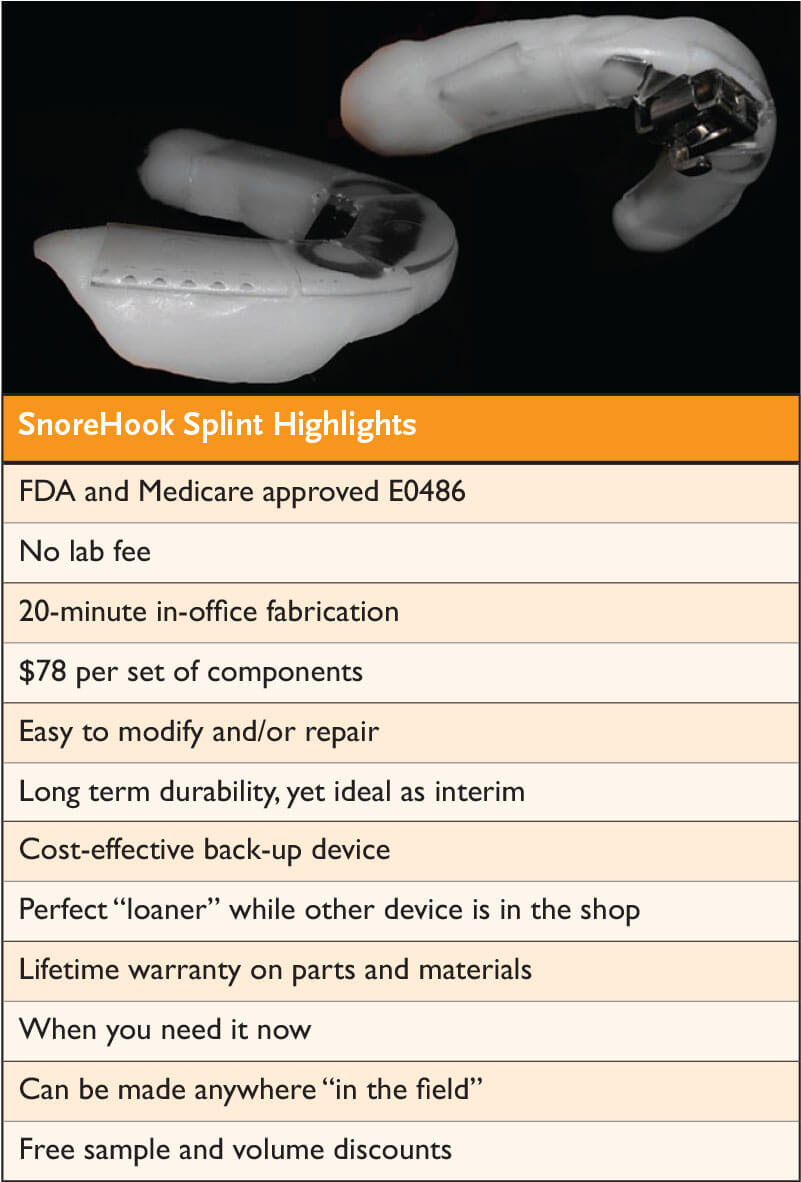 New Medicare-Approved Device is a Game-Changer for the Sleep Practice
New Medicare-Approved Device is a Game-Changer for the Sleep Practice
