Drs. Asim Roy and Brandon Canfield discuss Inspire, an implantable form of hypoglossal nerve stimulation, for people with OSA who are unable to use CPAP.
 by Asim Roy, MD and Brandon Canfield, DDS
by Asim Roy, MD and Brandon Canfield, DDS
Due to savvy marketing, ubiquitous multimedia ads, and efficacious treatment to support the buzz, Inspire is fast becoming synonymous with sleep apnea treatment in many households. Lack of detailed information and far worse – misinformation – abound about the procedure in some professional circles. Inspire isn’t a competing therapy that aims to cannibalize the therapeutic market. It is an innovative and wildly effective treatment that is only for a select patient profile. With this article, we aim to explain what Inspire is, who the prospective patient population is (and isn’t), and how dentists and Inspire doctors can collaborate to ensure that all patients get the treatment that’s best for them.
What Is It & How Does It Work?
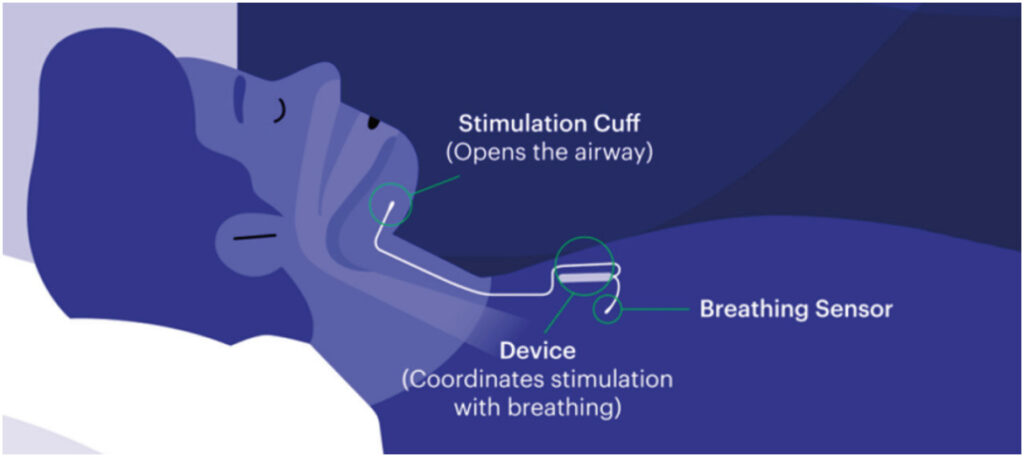
A major determining factor of upper airway patency during sleep is the activity of the genioglossus muscle (tongue muscle). Activation of this muscle via stimulation of the hypoglossal nerve is a creative new approach for treatment of obstructive sleep apnea (OSA). Hypoglossal nerve stimulation therapy is commonly referred to as Inspire, a reference to the name of the company – Inspire Medical Systems – that developed the treatment, which was approved by the Food and Drug Administration in 2014. Inspire therapy is an implantable treatment option for people with OSA who are unable to use or get consistent benefit from continuous positive airway pressure (CPAP). It’s a small implant that is inserted through two (previously three) small incisions during a same day outpatient procedure. When they go to sleep each night, Inspire patients turn the device on with their Inspire sleep remote.
Two leads are connected to the generator (battery). One lead senses when the patient inhales. The other sensor sits on the nerve that controls tongue movement and delivers mild stimulation that nudges the tongue forward, preventing the collapse that blocks the airway. This happens throughout the night – the system continues to keep the airway open, giving the patient a restful night’s sleep. The generator is expected to last 11 years before it needs to be replaced.
While the patient is sleeping, Inspire monitors every breath they take. Based on their unique breathing patterns, the system delivers mild stimulation to the hypoglossal nerve, which controls the movement of the tongue and other key airway muscles. By stimulating these muscles, the airway remains open during sleep. Inspire is controlled by a small handheld remote. The remote allows the patient to turn Inspire therapy on before bed and off when they wake up, adjust stimulation strength, and pause during the night if needed. Patients have full control over the device. It is essentially a pacemaker for the tongue.
Patient Criteria
Criteria for Inspire patient candidacy include:
- Moderate to severe obstructive sleep apnea (AHI of 15-65).
- Unable to use or get consistent benefit from CPAP.
- Not significantly overweight (ideally BMI < 35)
- Over the age of 18.
- Favorable airway by DISE (drug induced sleep endoscopy). DISE is a procedure that looks at a person’s airway under conditions that mimic sleep.
Does it Really Work?
The pivotal study of hypoglossal nerve stimulation was the Stimulation Therapy for Apnea Reduction (STAR) trial, which was authored by Patrick J. Strollo Jr., MD, et al and appeared in The New England Journal of Medicine in 2014. The trial included 126 patients with OSA who had difficulty initiating or maintaining continuous positive airway pressure (CPAP) therapy.
The stimulator was associated with a 68 percent reduction in the apnea-hypopnea index (AHI), from 29.3 events an hour to 9.0 events an hour at 12 months. Sixty-six percent of subjects achieved a reduction of at least 50 percent and an AHI of less than 20 events an hour. The AHI reduction was accompanied by improvements in daytime sleepiness and functional outcomes of sleep. The rate of serious adverse events was less than 2 percent.

Non-serious side effects included temporary pain at incision sites, transient tongue weakness, and tongue soreness. Tongue soreness improved over time with acclimatization, device reprogramming, or both.
Maintenance of upper airway stimulation therapy efficacy at three and five years in the STAR cohort has subsequently been reported. There is also the ADHERE registry which has over 2,100 patients enrolled in a cohort study sponsored by Inspire.
In total, over 8,000 patients have currently been implanted with the Inspire device. The growth has been tremendous. Nearly 150 new centers began offering Inspire therapy in 2020 which increases the total number of centers to more than 470 centers.
Additionally, Inspire recently conducted market research which revealed that most physicians did not believe Inspire therapy was covered by medical insurance. It is covered by most major providers – 64 total commercial insurance plans that provide coverage for 262 million members.
Alternatives to Inspire
People are not good candidates if they don’t meet the previously mentioned criteria. As you can deduce, this means many patients are not good candidates for Inspire. However, we still need to treat their sleep apnea.
There are many viable alternatives to CPAP or Inspire for these patients. Something many Dental Sleep Practice (DSP) readers are familiar with is oral appliance therapy (OAT) that uses a mandibular advancement device (MAD) to advance the jaw forward to achieve airway patency. There are other surgical solutions that can be helpful such as maxillomandibular advancement (MMA) and uvulopalatopharyngoplasty (UPPP).
Recently pharmacotherapy has been shown to be effective using two medications in combination that affect the upper airway (atomoxetine and oxybutynin). Recent technological developments of devices called iNAP (Somnics, Inc. Taiwan) and exciteOSA (Signifier Medical Technologies, London) both received FDA approval within the last year and can be effective in treating sleep apnea in certain cases. Conservative measures such as positional therapy and weight loss can also be effective. The key to this is not ignoring the disease but working in a multidisciplinary approach to treat the disease.
Inspire & Dental Sleep Medicine
Dentists have a vital role in this multidisciplinary approach in numerous ways. As mentioned above, dentists can treat patients who are not candidates for Inspire or CPAP with oral appliance therapy. As highlighted by Dr. John Viviano in the Summer edition of DSP, OAT has been shown to be as effective as CPAP in a recent meta-analysis.
Additionally, some patients may be non- compliant with their PAP due to their need for high pressure settings. These patients can have a MAD fabricated to wear in conjunction with their PAP to reduce the pressure setting and make them more compliant with their PAP therapy. In this same vein, some Inspire patients can have their voltage reduced or be better treated at their current voltage with OAT combination therapy. Keeping a lower voltage is more comfortable for patients and leads to less nighttime awakenings.
Dentists can also contribute to patient care by treating a minor but common side effect of Inspire therapy. Due to the tongue thrusts inherently caused by the hypoglossal nerve stimulator, some patients complain of tongue irritation from sliding along uneven edges of the patient’s teeth. To protect the tongues of these patients, a dentist can fabricate a vacuum-formed matrix to fit comfortably over the patient’s teeth to wear while sleeping. This eliminates the discomfort that can result from the tongue thrust against sharp, uneven lower incisors.
Dental practices play a crucial role in screening for sleep apnea. The American Dental Association released a policy statement which states assessing a patient’s risk for sleep related breathing disorders (SRBD) is part of a comprehensive medical and dental history for all patients. Once patients screen positive for SRBD, the dentists can refer to a physician for testing, diagnosis, and subsequent therapy thus increasing the dentist’s role in this multidisciplinary approach.
Dentists encounter patients that may be disqualified as OAT candidates for multitudinous reasons (e.g. severe periodontal disease, upper and lower dentures, acute TMD). These patients can be referred back to their physician to consider Inspire as a treatment alternative.
Likewise, many Inspire providers refer patients that are precluded from Inspire treatment to dentists for OAT. If a patient is not an Inspire candidate and does not respond to CPAP or OAT, we often consider pharmacotherapy. The iNAP device (negative pressure) or exciteOSA (daytime tongue stimulator) can be considered as adjunctive therapies. Occasionally supplemental oxygen can be beneficial. Other surgical options can be considered to reduce the burden of disease and the DISE often is helpful to make those decisions.
Patient Wellness is Our Shared Goal
Properly treating sleep apnea requires a multidisciplinary approach to patient care. Collaboration between ENTs and dentists is crucial for successful sleep apnea treatment plans for many patients. We share the primary goal of patient wellness. We must move away from the algorithmic, cookie cutter approach that considers the full swath of treatment options only after patients have first tried and failed PAP therapy. We need better phenotyping of these patients so we can administer optimal therapy sooner than later. Inspire should be considered for many of these patients as it has been proven to be highly effective for patients with moderate to severe sleep apnea that previously had limited options.
Besides technology like Inspire, dentists who treat SDB patients have many other tools integrated into everyday practice. Read Dr. Erin Elliott’s article about “Challenging the Status Quo.” https://dentalsleeppractice.com/challenge-the-status-quo/
 Asim Roy, MD, is the medical director of the Ohio Sleep Medicine Institute. Board certified in sleep medicine and neurology, he works in close collaboration with health care providers to deliver continuous and coordinated sleep medicine care to adult and pediatric patients. He is the author of various articles, chapters, and books, covering sleep and neurological disorders, restless legs syndrome, idiopathic hypersomnia, cataplexy, and REM sleep behavior disorder. Dr. Roy is a member of the American Academy of Sleep Medicine, American Academy of Neurology, American Medical Association, and Columbus Medical Association.
Asim Roy, MD, is the medical director of the Ohio Sleep Medicine Institute. Board certified in sleep medicine and neurology, he works in close collaboration with health care providers to deliver continuous and coordinated sleep medicine care to adult and pediatric patients. He is the author of various articles, chapters, and books, covering sleep and neurological disorders, restless legs syndrome, idiopathic hypersomnia, cataplexy, and REM sleep behavior disorder. Dr. Roy is a member of the American Academy of Sleep Medicine, American Academy of Neurology, American Medical Association, and Columbus Medical Association. After receiving his bachelor’s degree from The Ohio State University, Brandon Canfield, DDS, continued his studies at OSU earning his Doctor of Dental Surgery degree from The Ohio State University College of Dentistry. Dr. Canfield’s interest in sleep dentistry was sparked by his own suffering of sleep apnea that was relieved by wearing an oral appliance. He is a member of the Academy of Dental Sleep Medicine and is a Diplomate of the American Board of Dental Sleep Medicine. Dr Canfield is currently an Adjunct Assistant Professor in the Division of Restorative and Prosthetic Dentistry at The Ohio State University College of Dentistry.
After receiving his bachelor’s degree from The Ohio State University, Brandon Canfield, DDS, continued his studies at OSU earning his Doctor of Dental Surgery degree from The Ohio State University College of Dentistry. Dr. Canfield’s interest in sleep dentistry was sparked by his own suffering of sleep apnea that was relieved by wearing an oral appliance. He is a member of the Academy of Dental Sleep Medicine and is a Diplomate of the American Board of Dental Sleep Medicine. Dr Canfield is currently an Adjunct Assistant Professor in the Division of Restorative and Prosthetic Dentistry at The Ohio State University College of Dentistry.