Oral appliance billing can hinge on two codes, the E-code and the new K-code. Find out the difference, and how having two codes for oral appliances for obstructive sleep apnea — with and without a mechanical hinge — may pave the way for a broader range of choices for reimbursable appliances.
 by Rose Nierman, CEO, Nierman Practice Management
by Rose Nierman, CEO, Nierman Practice Management
The landscape of billing for oral appliances in dental sleep medicine is constantly shifting. As a dental office delving into the intricacies of dental sleep medicine billing, you’ve likely encountered the buzz surrounding the new “K” code, K1027. In this article, we’ll embark on a journey through oral appliance billing, and discuss some of the updates Nierman Practice Management has found.
Understanding the Obstructive Sleep Apnea Oral Appliance Codes
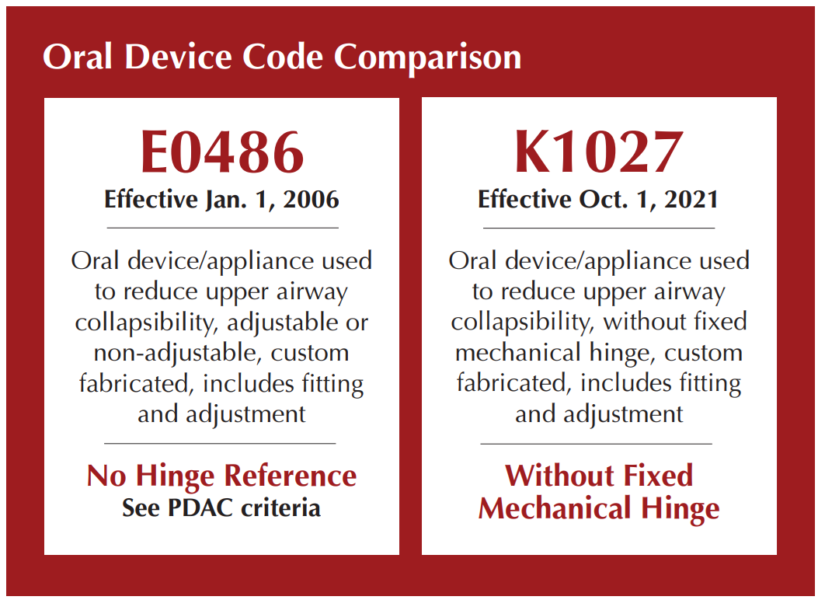
For years, we’ve relied on E0486, the established code for custom oral appliances. But now, enter stage left: K1027, the new code on the block. What sets this code apart? Well, it’s essentially E0486’s cousin, minus the mechanical hinge.
- E0486: Oral device/appliance used to reduce upper airway collapsibility, adjustable or non-adjustable, custom fabricated, includes fitting and adjustment
- K1027: Oral device/appliance used to reduce upper airway collapsibility, without fixed mechanical hinge, custom fabricated, includes fitting and adjustment
Why Do We Have Two Codes for Oral Appliances?
In 2012, Medicare added written criteria for a hinge relating to E0406 which has sparked considerable discussion about reimbursement. This stipulation was puzzling to many dental professionals as there was no evidence suggesting that a device with a mechanical hinge outperformed one without. The emergence of two codes for oral appliance therapy stemmed from the inability to amend the hinge criteria associated with E0486. As a result, the creation of a new code, K1027, tailored specifically for non-hinged appliances, was pursued as an alternative solution with the hope that it will pave the way for a broader range of choices for reimbursable appliances. Now, you may find yourself wondering, “Why didn’t they just amend the criteria?” It’s a valid question, and the answer lies in the intricacies of bureaucratic processes and the sometimes-perplexing decisions made by regulatory bodies.
What’s the Connection with the Hinge and Reimbursement?
The connection between a hinge and reimbursement for the E0486 started with PDAC. PDAC stands for Pricing, Data Analysis, and Coding. It’s an organization contracted by the Centers for Medicare & Medicaid Services (CMS) to provide coding and pricing determinations for durable medical equipment, prosthetics, orthotics, and supplies (DMEPOS). PDAC is crucial in establishing coding guidelines for medical equipment and supplies covered by Medicare. The PDAC criteria are embedded in Medicare’s Local Coverage Decision (LCD) for Oral Appliances for Obstructive Sleep Apnea, established in 2015. Here are the PDAC criteria:
To be coded as E0486, custom fabricated mandibular advancement devices must meet all the criteria below:
- Have a fixed mechanical hinge at the sides, front or palate; and,
- Be able to protrude the individual beneficiary’s mandible beyond the front teeth when adjusted to maximum protrusion; and,
- Incorporate a mechanism that allows the mandible to be easily advanced by the beneficiary in increments of one millimeter or less; and,
- Retain the adjustment setting when removed from the mouth; and,
- Maintain the adjusted mouth position during sleep; and,
- Remain fixed in place during sleep to prevent dislodging the device; and,
- Require no return dental visits beyond the initial 90-day fitting and adjustment period to perform ongoing modification and adjustments to maintain effectiveness.
A fixed hinge is defined as a mechanical joint, containing an inseparable pivot point. Interlocking flanges, tongue and groove mechanisms, hook and loop or hook and eye clasps, elastic straps or bands, mono-block articulation, traction-based articulation, compression-based articulation, etc. (not all-inclusive) do not meet this requirement.
PDAC has verified 98 appliances for E0486 and 23 for K1027, and they are continually adding additional manufacturers and laboratories to the PDAC list. To check for PDAC verified devices, visit DMEPDAC.com and enter the codes in the product classification area.
“You can navigate K1027 effectively and optimize reimbursement.”
What’s the Landscape Now?
With the introduction of these policy additions, numerous DSM providers are requesting coverage guidelines for both codes when making insurance verification calls. Nierman Practice Management recently tracked 200 verification calls to payers and received EOB data from several providers for K1027. This is what our sampling revealed:
- Many insurance plans indicated both codes as covered, although a few plans only have coverage for E0486.
- A major finding was that K1027 did not require preauthorization as often as E0486 with some of the insurers.
- In our billing sample, we saw a relatively consistent reimbursement range for E0486 whereas the reimbursement for K1027 varied with carriers. We saw lower payments here and there, but with some payers, we had higher reimbursements than with E0486.
It’s worth noting that many of these policy additions have taken place in recent months and are still evolving. January 2024 marked a significant milestone, with many payers incorporating K1027 into their policies.
What Should We Do Next?
- Optimize Verification Processes: It’s what you do upfront that counts. Verify insurance benefits for both K1027 and During a verification call, the biller should ask if preauthorization is required for each individual code.
- Optimize Billing Processes: Avoid billing oral device codes as morning repositioner appliances, which can inadvertently lower the allowed fees.
- Analyze Your Reimbursement Trends: Monitor and track billing your payments for E0486. If you bill any with K1027, track those EOB’s as well.
- Get your Ducks in a Row with Documentation: When billing new codes, create detailed SOAP narrative reports including appliance type and design.
- Educate Team and Providers: Keep your finger on the pulse of the continually shifting terrain of dental sleep medicine with educational courses and billing
- Engage with Professional Networks: Stay in touch with other providers and billers. Attend meetings to exchange insights and best practices regarding the implementation of K1027.
- Evaluate Patient Impact: Assess how the availability of K1027 impacts patient appliance choices and satisfaction. Consider any adjustments to typical treatment approaches accordingly.
By taking these steps, you can navigate the evolving landscape surrounding K1027 effectively and optimize reimbursement outcomes for your practice. For Medicare claims, a definitive shift may hinge upon Medicare’s establishment of an LCD for K1027.
The introduction of K1027 has ushered in a new era in dental sleep medicine, sparking discussions, challenges, and opportunities. As we adapt to new reimbursement policies and continue to advocate for patient-centered care, it’s crucial to stay informed, embrace change, and advocate for policies that prioritize patient choice and quality of care. With dedication, collaboration, and a commitment to excellence, we can ensure that dental sleep medicine remains at the forefront of improving patients’ lives.
Other New OSA Codes of Interest
New codes for Neuromuscular Electric Stimulation of the Tongue, (NMES) represent an innovative approach to managing mild OSA and snoring. Here’s a breakdown of the relevant codes and their descriptions:
- E0492: Power source and control electronics unit for the oral device or appliance used for NMES of the tongue
- E0493: An oral device or appliance specifically designed for neuromuscular electrical stimulation of the tongue muscles. It includes the device, the power source and control electronics units controlled by a phone application. This code covers a 90-day supply of the device.
NMES has been used for increasing and maintaining muscle strength and preventing atrophy in other areas of the body, particularly helping spinal cord injury patients walk. For breathing disorders, therapy involves sending electrical impulses to the tongue muscles, causing them to contract and strengthen. eXciteOSA® indicates this daytime therapy is designed to reduce airway blockage and alleviate symptoms of OSA and snoring. Patients typically undergo 20-minute sessions daily for six weeks, then maintain muscle tone by using the device one to four times a week afterward. Clinically proven results of symptom reduction are usually found within the six weeks onboarding process.
Given the novelty of NMES technology for OSA treatment, national insurance guidelines have yet to be established. Insurers are gradually becoming aware of these devices and may require additional information for coverage determinations on a case-by-case basis.
These new E-codes, alongside the K-codes, contribute to the evolving landscape of dental sleep medicine, offering practitioners comprehensive tools for managing sleep-related breathing disorders and improving patient outcomes.
Read about oral appliance billing and maximizing benefits of medical insurance in “Tips for Estimating and Presenting Out-of-Pocket Costs to Patients When Billing Medical Insurance,” here: https://dentalsleeppractice.com/tips-for-estimating-presenting-out-of-pocket-costs-to-patients-when-billing-medical-insurance/





 A pioneer of medical billing in dentistry, Rose Nierman is the CEO of Nierman Practice Management (NPM) and creator of DentalWriter Plus Software. For more than 30 years, Rose has taught dental practices successful and ethical medical billing through the iconic Successful Medical Insurance in Dentistry seminars. Contact NPM at 1-800-879-6468 or at
A pioneer of medical billing in dentistry, Rose Nierman is the CEO of Nierman Practice Management (NPM) and creator of DentalWriter Plus Software. For more than 30 years, Rose has taught dental practices successful and ethical medical billing through the iconic Successful Medical Insurance in Dentistry seminars. Contact NPM at 1-800-879-6468 or at