Dr. Allen J. Moses discusses how the X-Slant molar bite stabilization system represents a paradigm change that will revolutionize effective treatment.
An Ergonomic, Extremely Low-Cost, Multidimensional Monoblock Trial Sleep Appliance
by Allen J. Moses, DDS, DABCP, D.ABDSM
Introduction
The recent confluence of scientific advances in dental sleep medicine is resulting in a paradigm change that will revolutionize effective treatment.
- Digital registration of jaw position and computer-generated oral sleep appliances
- A Molar Stabilization device for multidimensional bite registration of oral appliances
- Recognition of the obsolescence and demise of mechanical adjustment mechanisms on oral sleep appliances
- Elucidation of the complex relationship of the anatomic components involved in oral airway maintenance during sleep
- Ergonomic design of oral sleep appliances
- Advent of noninvasive, accurate, safe, easy to use and low-cost home sleep testing
Digital Interarch “Bite” Registration and Computer-Generated Oral Sleep Appliances
The culmination of these advances presents to dental clinicians an ergonomic, extremely low-cost, multidimensional monoblock trial sleep appliance. It is fabricated using the following simple instructions:
- Using the X-Slant™ Molar Stabilization System, multidimensionally establish, then digitally scan and transmit the parallel, physiologic interarch jaw relationship (with minimum 0mm. interarch clearance) to the dental lab.
- Digitally scan and transmit upper and lower impressions of the patient’s dental arches to your dental laboratory.
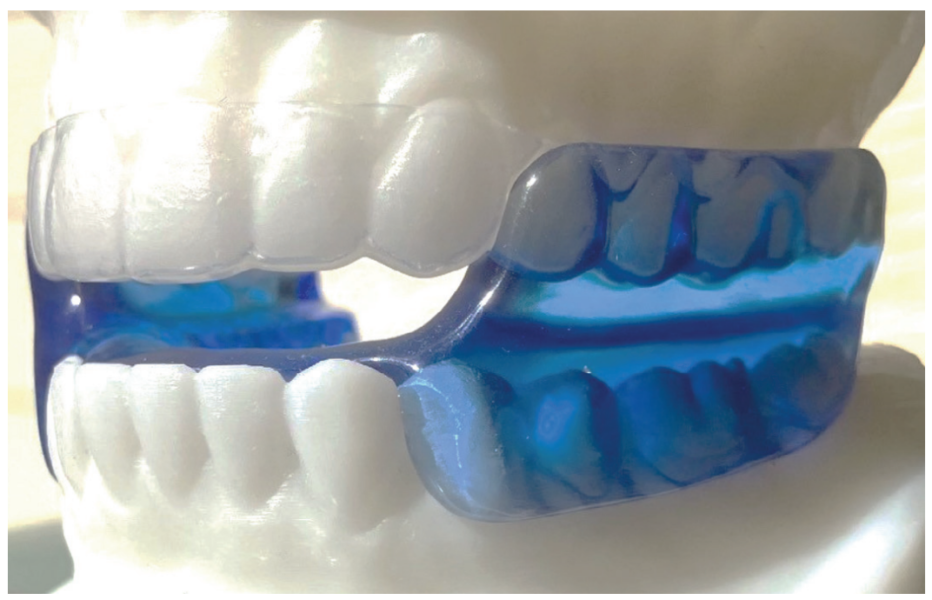
- Request a monoblock trial sleep appliance consisting of 0.40mm. acrylic upper and lower retainers fused into the trial interarch position by self-cured methyl-methacrylate (Figure 1).
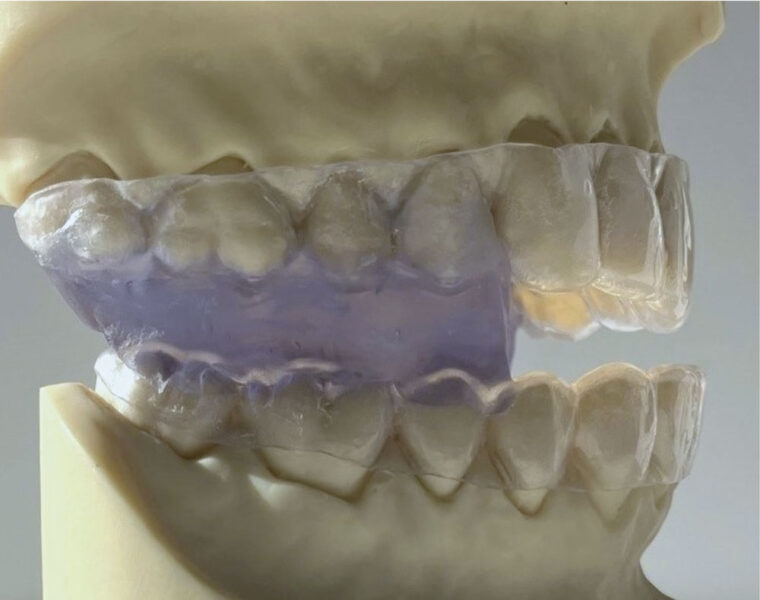
This article deals with how a simple ergonomic, extremely low-cost, generic multidimensional monoblock trial sleep appliance can enhance the quality and economics of future dental practice.
Molar Stabilization
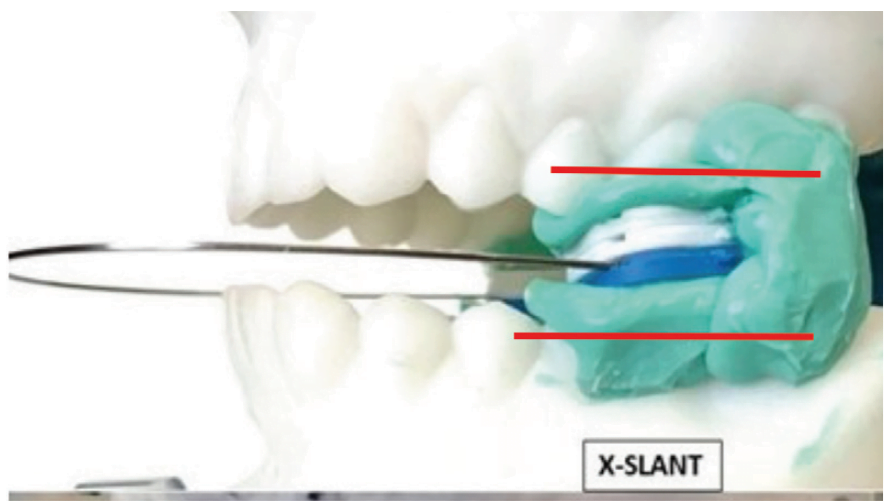
The interarch relationship for an intraoral sleep appliance is not a normally sustained biofunctional position for the mouth. The ideal interarch jaw position for an oral sleep appliance is optimal airway dilation and stenting with the lips comfortably closed. Dilation refers to getting the airway enlarged and stenting is keeping it open. Five specific dimensions need to be considered to determine treatment position for an oral sleep appliance: protrusive, lateral, vertical, cant and slant; and the device must be capable of registering asymmetric movement.1 X-Slant Molar Stabilization System is the only registration device commercially available that can properly establish all the necessary dimensional parameters.
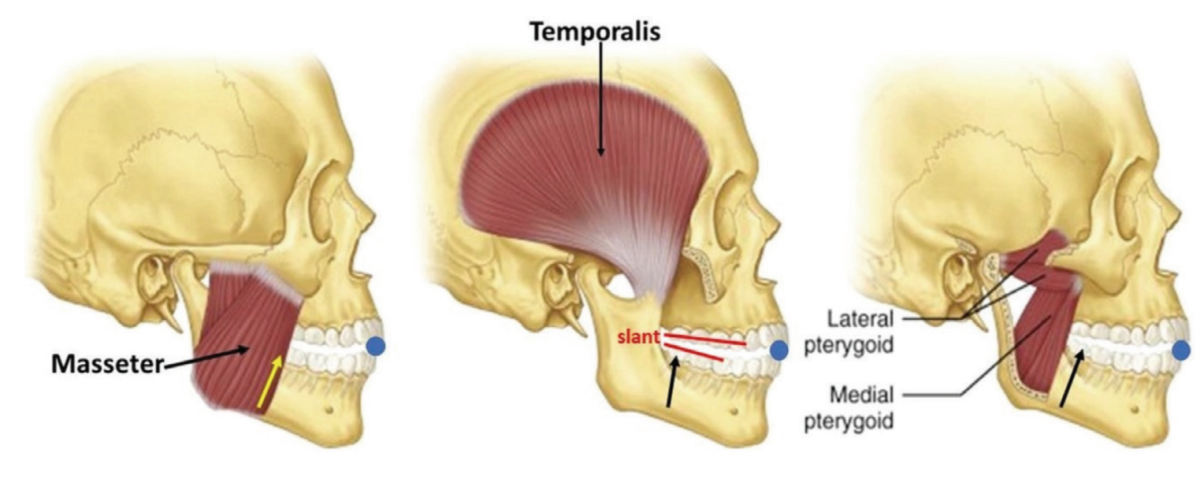
In an incisor stabilized interarch jaw registration, an acute angle seen between the plane of the mandibular teeth relative to the plane of the maxillary teeth and narrower at the posterior than anterior is referred to as “slant” (Figure 2). When the brain acts on the neural signal, “Close teeth on stabilizer”, to establish the interarch jaw relationship using an (anterior) incisor stabilization device, the focus of action is dictated by the elevator muscles in the molar area. With incisal stabilization there is a firm stop in the incisal area and no resistance in the molar area. The result is “slant”, an iatrogenic artifact.2 The negative physiologic consequence of slant, also referred to as “Elevator Muscle Squeeze”, is a reduced posterior oral airway diameter at the pharyngeal opening. There are no scientific bases to substantiate slant as a beneficial characteristic in an oral sleep appliance. To adequately achieve proper airway dilation at the oropharynx, slant may over-stent the mouth vertically at the anterior, making lip closure difficult, and consequently facilitate mouth breathing, another negative consequence. The good news is that slant is an avoidable phenomenon.
Molar stabilization devices, of which X-Slant is the prime example, focus the resistance at the molar area, and with no appreciable contractile force in the anterior, the result is parallel interarch planes, no slant and no diminution of mouth volume at the pharyngeal opening. The substantiating science is applied human anatomy.
The unique and inherent feature of molar stabilization devices such as X-Slant is that mandibular molar teeth are able to freely slide anteriorly, posteriorly and laterally. X-Slant takes up no tongue space, does not interfere with tongue movement, offers no arbitrary mechanical interference to jaw movement and accurately accommodates natural asymmetric and multidimensional jaw movement. Facilitating the full range of multi-dimensional movement to establish the optimal position for appliance fabrication is essential for biologic compatibility, comfort and optimal oropharyngeal positioning of a sleep appliance.
Obsolescence of Mechanical Adjustment Mechanisms
Unidirectional movement does not describe how airway dilation occurs with use of an oral sleep appliance. Optimal airway volume is based on multidimensional positioning. Unidirectional however, describes the adjustment mechanisms in virtually all mechanically advanceable oral sleep appliances. Any proposition that the neurologically coordinated movement of over 20 muscles moving in multiple directions with unpredictable firing sequences, anchored and guided by the irregular, asymmetric shapes of the hyoid bone, condyles and glenoid fossae could move in tandem and in a straight unidirectional dimension that can be set arbitrarily in a dental lab or by a clinician is contrary to any known anatomic principles3 and has no more credibility than guesswork.
Current mechanical adjustment mechanisms consisting of tubes, straps, set-screws, rubber bands, hinges, hooks and springs are unidirectional and do not replicate human condylar movement. Built-in adjustment mechanisms in oral sleep appliances are not specifically pre-calibrated for the direction necessary to achieve optimal airway dilation/stenting. Built-in mechanical expansion mechanisms are unidirectional, never having concurrent multi-dimensionality, almost universally horizontal in direction, very rarely vertical or programmed to register asymmetric movement. The presence of a unidirectional mechanical adjustment mechanism in an oral sleep appliance limits its characterization to temporary or trial device, and it will remain as such until it is objectively validated by HSAT.
Oral Sleep Appliances that Allow TMJ Movement During Sleep and Pharyngeal Dilation
The old idea that oral appliances are merely mandibular advancement devices (MADS), moving the tongue forward to prevent its collapse on the airway is an oversimplification. It does not account for the dilation of the sides and back of the pharynx. The pharyngeal constrictors are not attached to either the tongue or the mandible. The multidimensional airway dilation shown cannot be accounted for solely by active anterior movement of the mandible and the attendant passive pull-forward of the tongue base. Finding a mouth position that dilates the throat has a complex effect on the musculature of the entire airway.4
Maintaining a dilated airway during sleep has also been shown to be problematic. Mandibular movement during sleep changes the shape of the oropharynx. Intraoral sleep appliances that allow mandibular movement do not maintain a stable, stented airway position during sleep. Airway size changes multidimensionally with repositioning of the anatomic parts of the oral apparatus, i.e. lips tongue, soft palate, pharynx and hyoid bone.
Note: The Monoblock Generic Custom Sleep Appliance shown in Figure 1. maintains a stable oral airway during sleep.
Elucidation of the Complex Relationship of Anatomic Components Involved in Oral Airway Maintenance During Sleep
The combined interaction of the collapsible pharynx, tongue, temporomandibular joints, soft palate, hyoid bone, musculature, head posture, and nervous system to optimally dilate and stent the airway during sleep has been discussed in a previous paper.5 It is a movement so complex that any concept of definitively effecting a multidimensional airway dilation by advancing a single, unidirectional mechanism is an oversimplification.
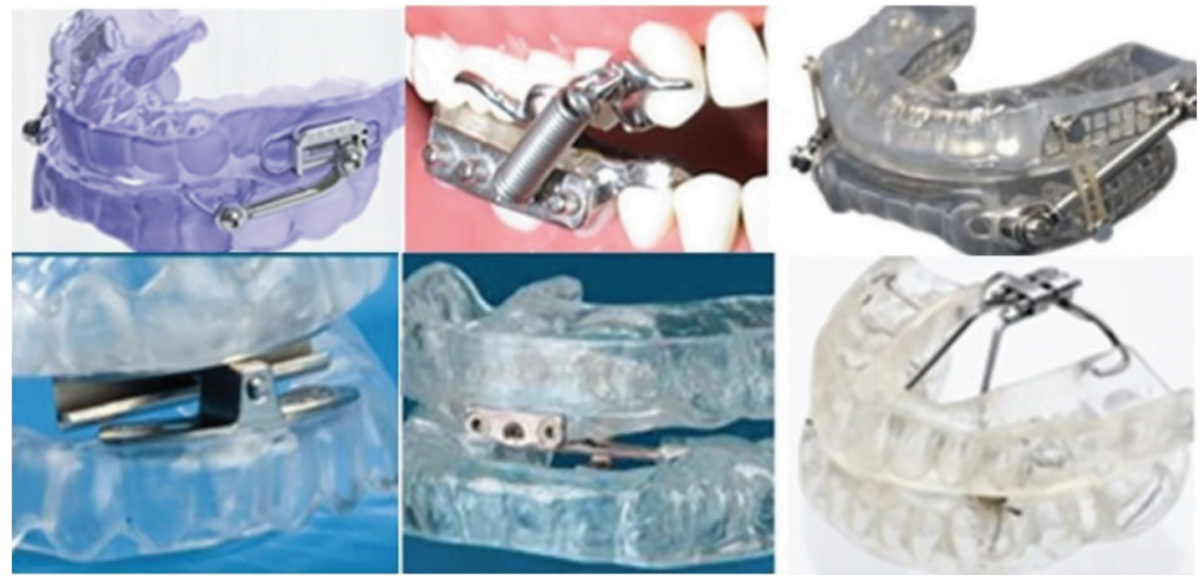
Ergonomic Design of Oral Sleep Appliances
Ergonomics is the process of designing products so they optimally perform and fit, enhancing their efficiency. The field of ergonomics considers anthropometry, physiology, biomechanics, design, kinesiology, comfort, ease of use, esthetics and satisfaction. Ergonomically designed oral sleep appliances integrate these principles into overall performance. Examples of non-ergonomic oral appliances are those that compromise tongue and mouth space, such as those with wires or hardware that prevent the tongue from resting on the palate, those with restrictive anterior devices that prevent the tongue from reaching the lips or those whose vertical anterior height prohibits comfortable lip closure.
Custom-made oral appliances are those in which all components are fabricated to the unique personal proportions of the individual’s anatomy. Store-bought mechanical parts built into an oral appliance do not make it custom or ergonomic. A unidirectional adjustment mechanism on an oral appliance determines definitively that the device is not custom and not ergonomic.
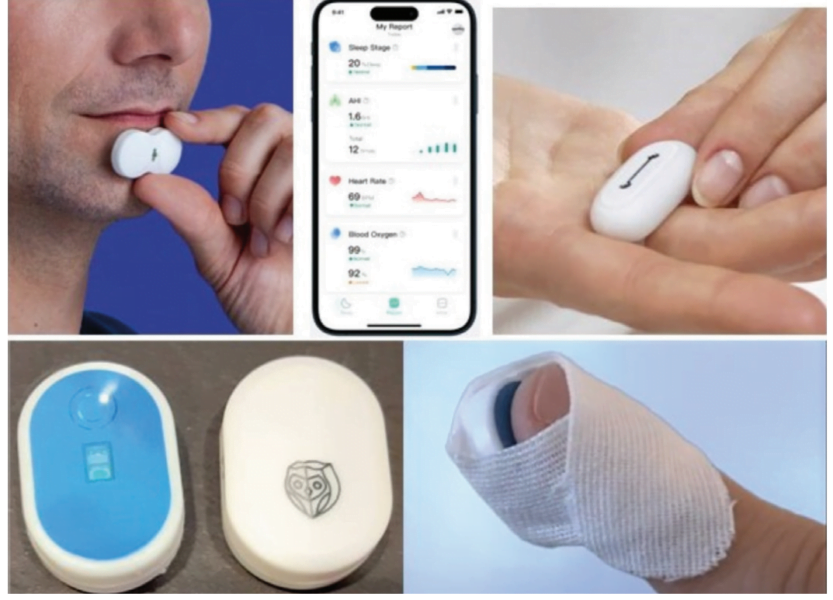
Non-invasive, Accurate, Safe, Easy To Use, Low-Cost Home Sleep Testing
The clinical practice guideline for diagnostic testing for adult sleep apnea by the American Academy of Sleep Medicine (AASM) formulated a strong recommendation that home sleep apnea testing (HSAT) is an appropriate diagnostic testing option for uncomplicated adult patients who are at increased risk of moderate to severe sleep apnea.6 HSAT devices that analyze changes in peripheral arterial tone (PAT) in combination with actigraphy and blood oxygen saturation (SpO2) have been determined to be adequate to diagnose OSA in patient populations with a high pretest probability.7
Typical systems consist of a small optical sensor device which is placed on the finger, fingertip or chin. The sensors are designed to be easily self-applied by the patient. The sensor acquires accelerometer data and reflectance-based photoplethysmography (PPG) signals. The software derives SpO2, PAT, pulse rate and other biophysical parameters recommended by The AASM Manual for the Scoring of Sleep and Associated Events for home sleep apnea testing. Collected data from the sensor is continuously streamed to a user’s smartphone via Bluetooth then processed by a state-of-the-art sensor’s data algorithm and results are displayed via an on-line dashboard for doctors to login to on their smartphone.
The main limitation of PSG has been its prohibitive expense which determined that it be a single-night in-laboratory, clinician attended sleep study. Repeated unattended sleep testing in the home environment is now recognized as a more thorough approach. With the costs for some HSATs now being below $100 US dollars,9 good clinical practice would seem to mandate home testing the efficacy of oral asleep apnea devices as many times as the clinical guidelines determine is reasonable, to achieve successful treatment. Dentists trained to treat sleep apnea should be knowledgeable to read an HSAT and titrate an appliance when necessary.
Discussion
- Reorientation of the clinical protocol to “Validate Before Delivery” or “Try Before You Buy”, really does not introduce new science but is an example of a major paradigm change that calls for rearranged integration of the several existing parts.
- Present technology as well as ergonomics, cost efficiency and science create the possibility to objectively evaluate the efficacy of a trial sleep appliance before incurring the expense of laboratory fabrication of the permanent appliance.
- Use of a simple, new, low-cost, molar bite registration system (such as X-Slant™) with no mechanical restriction of natural asymmetric movement, and no tongue interference, establishes the multidimensional parameters of vertical, lateral, protrusive cant and eliminates slant completely, with a high degree of accuracy.
- The trial sleep appliance presented in Figure 1 can be handcrafted, or inexpensively fabricated, yet virtually replicates or clones the function of the permanent oral sleep appliance shown in Figure 5.
- It may be necessary to test more than one trial device, but the low cost of the monoblock device shown in Figure 1 is so nominal several can be tried.
- Built-in mechanical adjustment mechanisms in oral sleep appliances such as tubes, hinges, springs, screws and straps are presented as being impractical, obsolete and not ergonomic because their movement is unidirectional and does not replicate multidimensional TMJ movement.
- The low-cost trial device can be tested, objectively validated and manufactured before delivery of a “final version”.
- Additional recognized ergonomic factors for validation before delivery are comfort, simplicity, very low cost and durability.
- The recent innovation of accurate, efficient, low cost and simple home sleep apnea testing technology based on easy, self-applied disposable sensors and smartphone software is the culminating event for the reality of a “Try it before you buy it” sleep dentistry protocol,
Conclusion
The demise and obsolescence of the traditional concept of non-custom, non-ergonomic, expensive, unidimensional, appliances having built-in adjustment mechanisms and being represented at delivery as more than a trial device was thoroughly explained. The new paradigm, that an inexpensive “Trial” oral sleep appliance, based on an improved bite registration system and incorporating the confluence of scientific advances discussed, can simultaneously capture all multidimensional parameters, be made from scratch, tested, and validated before fabrication of the permanent nonadjustable appliance just makes more sense.
Addendum: Dentists are offered a complimentary sample of X-Slant, the molar bite stabilization system discussed in this article by sending their complete mailing address to info@themoses.com and simply writing “Yes, send me one” in the subject line.
*The device shown in Figure 1 is a generic device, not trademarked, not patent protected, not patentable by its common use and does not require FDA 510K clearance. The dental lab has no license fee or royalty or use a proprietary part so the expected charge for fabricating this device might be $80 – $110. A dentist’s work order to the lab should accurately describe the structure of the device and specify that materials used be FDA approved for safety.
FDA 510K Clearance is required for U.S. manufacture and/or sale of oral sleep appliances using proprietary, trademarked, prefabricated or non-custom parts. Approval is based on demonstrating safety by use of specified FDA approved materials and confirmation of “substantial equivalence” in structure to two previously 510K cleared oral sleep devices.
**Figure 5 shows a Moses Alpha™, a definitively effective, proprietary patented, trademarked, licensed and FDA 510K cleared oral sleep device. Its cost from Modern Dental Lab USA and Micro Dental Labs is $250 U.S. If the device needs to be remade, the upper component is re-used and the lower component is replaced for a charge of $175.
Read more about why it is important to look for an interarch jaw registration that eliminates slant and facilitates normal shift like the X-Slant molar bite stabilization system here: https://dentalsleeppractice.com/bigger-picture/interarch-jaw-registration-devices-for-oral-sleep-appliance/
- Moses AJ, Interarch Jaw Registration Devices for Oral Sleep Appliances: Consequences of Anterior vs Posterior Stabilization. Dental Sleep Practice, Winter 2022, p.30-35
- Moses AJ, Interarch Jaw Registration Devices for Oral Sleep Appliance: Part 2, Shift Happens. Dental Sleep Practice, Spring 2022, p.16-19
- DuBrul EL, Sicher’s Oral Anatomy by DuBrul. CV Mosby, Philadelphia 7ed, 1980
- Schwab RJ, Kuna SJ, Remmers JE, Anatomy and Physiology of Upper Airway Obstruction 82: 983-1000; In Princ and Pract Sleep Med; 4ed. Kryger, Roth, Dement, 2005 Elsevier Saunders, Philadelphia
- Moses AJ, Relating Oral Physiology to Sleep Apnea Positioning. Dental Sleep Practice, Winter 2023, p.44 – 48
- Kapur VK, Auckley DH, Chowdhuri Set al.Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med; 2017;133:479-504
- Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med; 2007;37:737-747
- Berry RB, Brooks R, Gamaldo CE,et al. for the American Academy of Sleep Medicine The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications Darien, IL: American Academy of Sleep Medicine Version 2.4; 2017.
- https://www.sleepon.us/go2sleep/?utm_source=google&utm_medium=cpc&utm_campaign=19668111319&gclid=Cj0KCQjw1aOpBhCOARIsACXYv-fN0esw-xnX6wTMleBa3Gv5A4wiSjtvOTChmX4aQO153dQBUZ_jy4caAlL5EALw_wcB
 Allen J. Moses, DDS, DABCP, D.ABDSM, was in private dental practice for 48 years in Chicago Illinois and assistant professor at Rush University Medical School in the Department of Sleep Disorders and Research for 13 years. He holds three US Patents for intraoral sleep devices and a Patent Pending for a shim system for registering the posterior interarch jaw relationship for intraoral sleep appliances. He was a member of the United States Food and Drug Administration Dental Products Panel for 10 years, has authored a book on temporomandibular disorders and over 30 scientific papers on TMDs and sleep dentistry. He was book review editor of Cranio for 10 years.
Allen J. Moses, DDS, DABCP, D.ABDSM, was in private dental practice for 48 years in Chicago Illinois and assistant professor at Rush University Medical School in the Department of Sleep Disorders and Research for 13 years. He holds three US Patents for intraoral sleep devices and a Patent Pending for a shim system for registering the posterior interarch jaw relationship for intraoral sleep appliances. He was a member of the United States Food and Drug Administration Dental Products Panel for 10 years, has authored a book on temporomandibular disorders and over 30 scientific papers on TMDs and sleep dentistry. He was book review editor of Cranio for 10 years.