
by Jerry Hu, DDS, David Kuhns, PhD, Sung Kim, BS, Len Liptak, MBA, Laura Sheppard, CDT
Background
Obstructive sleep apnea (OSA) is gaining tremendous attention as an acute public health concern. A study by the Institute of Medicine reports that 50 to 70 million Americans suffer from disorders of sleep and wakefulness1. The long-term effects of sleep loss and disorders have been associated with increased risk of hypertension, diabetes, obesity, depression, heart attack and stroke. One in five car accidents are associated with driver sleepiness. The American Academy of Dental Sleep Medicine notes that over 18 Million Americans have OSA2.
Due to patient compliance issues that limit the effectiveness of the gold standard CPAP therapy3, the mandibular advancement devices (MADs) prescribed by dentists are playing an increasingly important role in the treatment of OSA.
Although many studies confirm the effectiveness of MADs, opportunities exist to optimize the design. Along with patient comfort, customization to the patient’s dentition, optimizing mandibular advancement intervals, minimizing the bite opening, and simultaneous advancement of the tongue and mandible are considered some of the key elements for treatment success with mandibular repositioning devices4.
The New MicrO2 Sleep Device
The new MicrO2 Sleep Device (MicroDental Laboratories, Dublin, CA, Patent Pending) is designed to address many of the known opportunities for optimizing the performance of MADs. The MicrO2 is the first sleep device made from a control cured dental grade poly methyl methacrylate (PMMA) material. This material is less porous, allowing the MicrO2 device to be stronger and more biocompatible than MADs made from traditional, cold cured PMMA material. Because the device’s material is stronger, it can be made smaller and more comfortable than traditional MADs. The enhanced material strength also provides the dentist with more treatment flexibility when it comes to optimizing the vertical bite opening.
MicrO2 is also the first precision milled MAD. Precision milling, as opposed to manual fabrication, offers advantages with respect to accurately mirroring the patient’s dentition, delivering the prescription consistently, and making it easier to replace if a device is lost or damaged. The CAD/CAM process enables a new titration method utilizing precise combinations of upper and lower arches, each with different fin offsets, designed to achieve the doctor’s prescription. With enhanced retention made possible by precision milling, ball clasps are optional and the MicrO2 device also features a lingualess design that creates more room for the tongue.
Another noteworthy feature is the 90 degree dorsal fin angle. The 90 degree fin angle is designed to hold the jaw forward in the prescribed position even when the mouth opens during sleep.
 Case Report: Overview
Case Report: Overview
Though the potential benefits of the MicrO2 may intrigue many dentists who are practicing dental sleep medicine, the compulsory question remains whether or not the device can deliver clinically efficacious treatment outcomes. For the purpose of this case report, efficacy is defined according to three treatment success thresholds that are commonly accepted in the field of dental sleep medicine5. The first success threshold is a post treatment apnea hypopnea index (AHI) score of less than 5. The second is a post treatment AHI score of less than 10. The third is a post treatment AHI score improvement (reduction) of 50% of better, if not exhibiting an AHI score below 10.
This single center case report (Jerry C. Hu, DDS, Soldotna, Alaska) is comprised of seven patients who received a MicrO2 Sleep Device for the treatment of OSA. Each patient was screened for OSA in the practice and completed a home sleep test (Watch-Pat, Itamar Medical, Franklin, MA). Each patient was diagnosed with OSA by a medical doctor who prescribed a MAD as either a supplement or alternative to CPAP. After being fitted with the sleep device, each patient repeated the home sleep test to confirm therapeutic efficacy.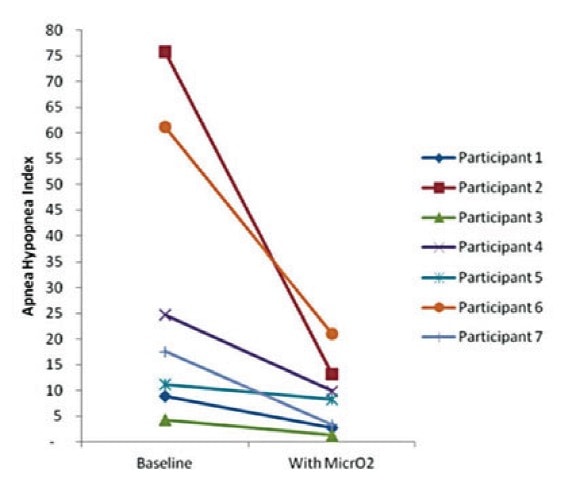
Case Report: Sample
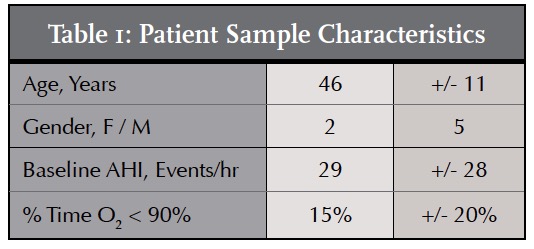
Although the sample size for this case report was modest (n = 7), the diversity of the participants is noteworthy. The age of the participants ranged from 22 years of age to 55 years of age, with a mean age of 46. Two of the patients were female. Five of the patients were male. The mean baseline AHI Score was 29. Two patients were diagnosed with severe sleep apnea, having baseline AHI scores greater than 30. Two patients presented with moderate OSA, AHI less than 30 but greater than 15, and three were diagnosed with mild OSA, baseline AHI scores of less than 15. The mean percentage of time with an O2 level less than 90% was 15%.
The sample was comprised of a mix of newly diagnosed patients, patients who abandoned CPAP therapy for various reasons, and patients who needed to replace a pre-existing MAD.
Case Report: Results
Seven of the seven patients fitted with the MicrO2 Sleep device achieved at least one of the three AHI score success thresholds. Three of the seven patients exhibited post-treatment AHI scores below 5. Five of the seven patients exhibited post-treatment AHI scores below 10. Six of the seven patients experienced an AHI score improvement of greater than 50%. One of seven patients did not achieve an AHI improvement of > 50%, however this patient did achieve an AHI score below 10.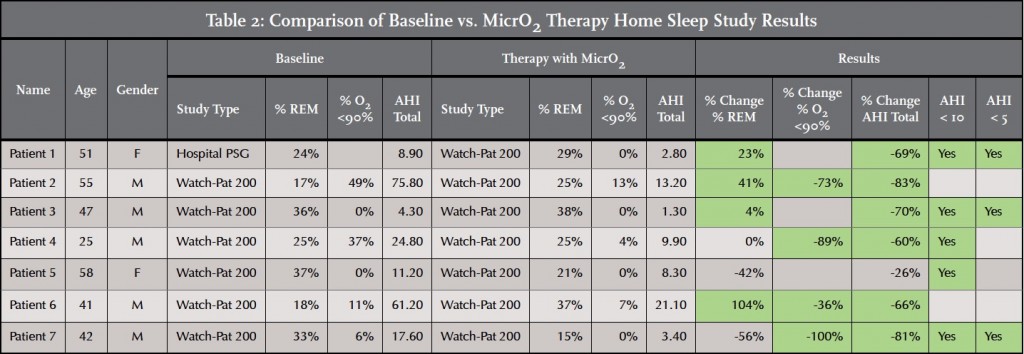
Four of the seven patients experienced an improvement in % REM sleep. One of the seven patients experienced no change. % REM sleep declined for two of the seven patients. However REM sleep time may improve with additional titrations.
Seven of the seven patients were able to achieve a % O2 <90% score that was either zero or a significant improvement relative to the baseline score. Four of the patients achieved a % O2 <90% score of zero.
It is important to note that the post-treatment AHI scores reported in this study reflect the initial mandibular repositioning with the MicrO2 Sleep Device as represented by the initial target bite. Further improvements in AHI scores and REM sleep are possible as the practice continues to optimize the calibration of the airway by titrating the device.
One of the more rewarding results came from a mild OSA case which exhibited AHI, RDI and ODI scores under 2 while wearing the MicrO2 Device. This patient is a shift worker and his spouse reported that his somnolence improved noticeably. Moreover, his REM sleep improved significantly and he felt as if he had slept for a few hours when he actually slept for over 7 hours without interruption. He reported no bite, occlusion, or TMJ-related issues.
Conclusion
Sleep apnea is a growing public health concern that represents an opportunity for dentists to provide important new type of care to their patients. The findings of this single-center case report of seven patients confirm that the new MicrO2 Sleep Device is a viable option for treating patients who are diagnosed with obstructive sleep apnea. Further research is required to evaluate additional potential benefits of this new MAD with respect to patient compliance and mitigation of MAD side effects. The unique design features may yield efficiencies in treatment protocols for the dental office.
For More about Sleep Apnea Diagnosis and Treatment
Before practicing dental sleep medicine, the authors encourage dentists to participate in at least one of the numerous continuing education opportunities available on the topic of Sleep Disordered Breathing. The Las Vegas Institute, The American Academy of Dental Sleep Medicine and The Pankey Institute are among the leading providers of continuing education courses on the practice of dental sleep medicine. For more information, contact these institutions and organizations, or contact MicroDental Laboratories.
Stay Relevant With Dental Sleep Practice
Join our email list for CE courses and webinars, articles and more..

