In our Cover Story, Wayne Wagner shows the evolution of some oral appliance therapies, and how the Slow Wave DS8 led to the launch of the new K1027 billing code.
by Wayne Wagner
Ten years ago, 80% of OSA sufferers were undiagnosed, and the same is true today.1,2 Ten years ago, oral appliance therapy had de minimis market share as a treatment for Obstructive Sleep Apnea. Today, even with the implosion of the CPAP industry due to the Philips recall and their subsequent exit from manufacturing the devices, Oral Appliance Therapy (OAT) still has an insignificant market share worldwide. Fortune Business Insights said the sleep apnea device market was worth $9.17 billion in 2023.3 Still, if you add all the revenue reported by oral appliance manufacturers, they don’t approach 5% of this number.
The Medical Innovation Challenge
Before 2021, oral appliances treating OSA had to meet a strict, seven-bullet product definition established by the Centers for Medicare/Medicaid (CMS) for how Mandibular Advancement Devices (MAD) should operate4 to get insurance reimbursement. The last two requirements imposed rigid design specifications that did little to enhance patient choices or improve efficacy:
- Have a fixed mechanical hinge at the sides, front, or palate; and,
- Incorporate a mechanism that allows the mandible to be easily advanced by the beneficiary in increments of one millimeter or less.
The dental sleep medicine industry was unhappy about the billing code restrictions but did nothing in a coordinated fashion to try to change them. They just lived with the antiquated billing code and the low reimbursement rates that accompanied it.
Billing Code K1027
In 2021, Slow Wave, Inc. did what the Dental Sleep Medicine industry could not do and gave CMS concrete reasons to introduce a new billing code. The introduction of the Slow Wave DS8 utilized a host of new design and manufacturing innovations that the dental community had embraced. CAD/CAM, 3D printing, and milling have become pivotal technologies in digitization. This shift enabled highly accurate digital scanners to replace traditional impression molds that often-required time-consuming adjustments.
The materials used in the new production processes had already received FDA clearance and allowed for the fabrication of teeth, bridges, and custom oral appliances with precision tolerances as tight as 1/100th of a millimeter. This digital revolution required a billing code that could evolve alongside technological advancements, and CMS agreed that the Slow Wave’s DS8 was ideal for launching the new K1027 billing code. Where the industry had failed to create change, a single product introduction succeeded. The product definition of K1027:
“Oral device/appliance used to reduce upper airway collapsibility, without fixed mechanical hinge, custom fabricated, includes fitting and adjustment.”
This more inclusive product definition has resulted in more than 27 products being approved under the new billing code in the three years since it was established by CMS.5
However, the fight for K1027 is far from over, and the industry needs to pull together to get CMS to issue both a benefit category and fee schedule so that both CMS and private insurers can reimburse the code. Until then, medical billing is not cost-effective, as the time and effort involved can exceed the appliance’s value. In addition, the reimbursement rate for billing code E0486, if applied to the new billing code K1027, will continue to suppress profitability in a historically highly unprofitable industry. This contrasts with Hypoglossal nerve surgery prescribed by physicians that gets reimbursed $50,000+ by CMS for a lower efficacy treatment.

The Bruxism/OSA Connection
The medical and dental communities have long suspected that both OSA and sleep bruxism may be concurrent in an individual. According to various research studies, OSA affects somewhere between 9% to 38% of the population.6 Sleep Bruxism (SB), a repetitive jaw muscle activity that manifests as clenching or grinding the teeth, is present in 5.5% to 8% of adults.7,8 The overlap between them is significant according to multiple studies, though. In one study of 147 patients, sleep bruxism was found to be present in 33.3% of the OSA patients studied. In another Japanese study, SB was found to be present in 48% of OSA patients.9 The question of why the overlap is still debated, but the Japanese study demonstrated positive correlations between “phasic-type sleep bruxism events and OSA desaturation, and microarousal event indices, suggesting that OSA might be a high-risk factor for sleep bruxism.”
OAT Mainstreamed for All Dentists
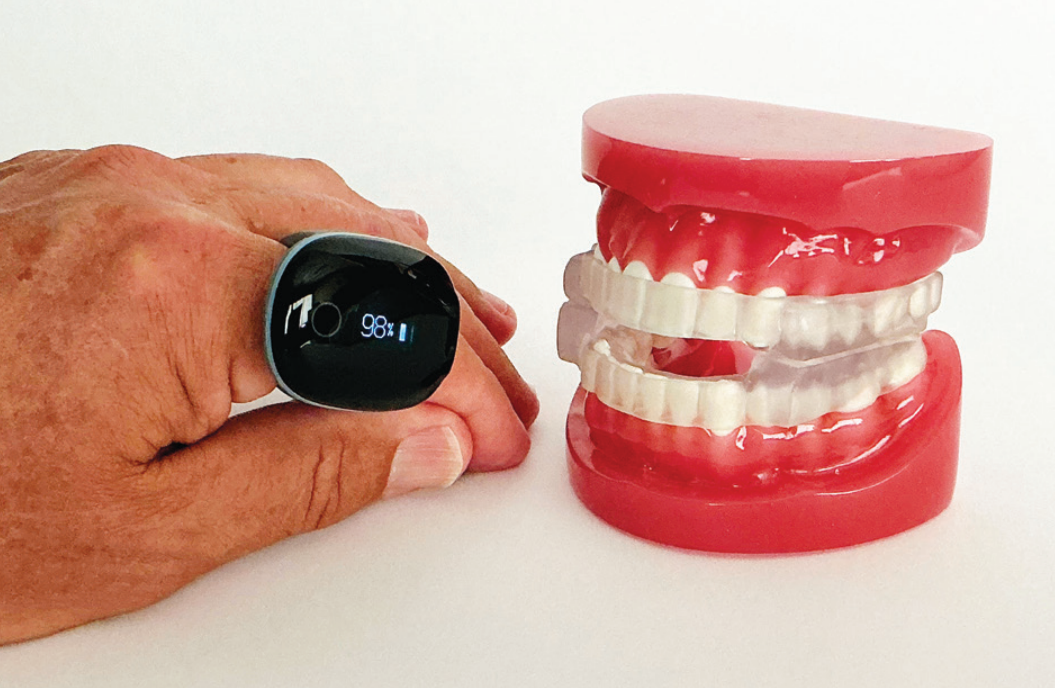
In 2017, the first oral appliance was dual cleared for OSA/Bruxism by the FDA. The LUCO Hybrid was initially cleared for bruxism in 2015 [510(k) # K16047] and, based on clinical data, cleared for OSA in 2017 [510(k) # K130797]. However, no one understood the implications of what this could do for the dental industry. In July 2024, the FDA dual cleared the Slow Wave DS8 for the treatment of OSA/Bruxism K191320 and K240463. The product, now marketed as BROSA (Bruxism, Retainer, OSA), is prescribed by dentists for a bruxism condition that shares a significant overlap with OSA.
Now, dentists can prescribe a product to treat a patient’s bruxism in just one visit and have treatment within days. The patient can also simultaneously monitor its impact on their sleep patterns. This new model makes it profitable for the dentist to make OAT a mainstream product.
Most existing oral appliances for bruxism are only on the top or bottom teeth and focus on mitigating the effects of the condition – such as reducing teeth from being ground down – rather than addressing the condition itself. This means that most mouth guards can’t really help bruxism, let alone serve as a treatment for other sleep conditions like OSA.
But what makes devices like BROSA effective for both OSA and bruxism? Two key factors are the vertical opening of the mandible and making more room for the tongue.
Traditional devices advance the mandible forward in millimeter increments to open the airway. Still, research has shown that incorporating more vertical opening and making more room for the tongue allows for less forward movement of the mandible. This can reduce or eliminate the need for a morning repositioner, as the jaw remains in a more relaxed state during sleep. This relaxed state is not only beneficial for OSA but also ideal for treating bruxism.
A clinical trial in Chile provided further evidence of this. The study compared three groups, each fitted with an occlusal device that opened the jaw to varying degrees: 1mm, 4.25mm, and 8.25mm. The results showed a significant reduction in masseter muscle tension in the groups with 4.25mm and 8.25mm openings, with the best results in the 8.25mm group.11 This vertical opening helped relax the muscles responsible for bruxism events.
The Slow Wave BROSA device further enhances this relaxation thanks to how well the Formlabs material glides while using a ramp system that allows for a very smooth natural horizontal movement during sleep. The innovative ramp mechanism ensures that the more a patient bites down, the more the ramps gently advance the mandible forward. This system allows the body to find its ideal jaw position naturally during sleep. Initial use of the BROSA device in patients enabled 24 of 25 to achieve normal breathing levels of fewer than 5 AHI events per hour. These outcomes were observed across patients with mild, moderate, and severe OSA.
New technology allows dentists to treat bruxism and patients to monitor their OSA.
O2 rings from Wellue and SleepImage™ and watches from both Apple and Samsung mimic, with great accuracy, the oxygen and pulse readings from home sleep study devices. These devices can give some of the 80% undiagnosed their first clue that a formal sleep study might be warranted. In September 2024, the Apple Watch was even FDA cleared under new product code QZW as an “Over-the-counter Device to Assess the Risk of Sleep Apnea.”10 The SleepImage System is FDA-cleared to aid clinical diagnosis of sleep disordered breathing in children as young as 2 years-old, adolescents, and adults. For those previously diagnosed, nightly monitoring of their sleep provides clues when intervention from a sleep doctor might be warranted.

A Dentist-led Solution for Sleep Disorders
For Dr. Robert D’Alfanso, DDS, at Lakeway Cosmetic and Family Dentistry near Austin, Texas, innovation has drastically changed his 20-plus year dental practice. “I used to wait weeks or months for implants to come in from a dental lab. Now, I have a local lab, and my patients get better and more immediate service.” But like most general dentists, sleep dentistry is something that Dr. D’Alfonso has been reluctant to adopt. “Between the physician referrals and medical billing, treating OSA has been a non-starter for me. Chasing down medical insurance companies or checking on the status of a sleep study diagnosis is not productive time for our busy team.” Dr. D’Alfonso is not alone in his hesitancy. In a survey conducted by MedMark Media in December 2020, when dentists were asked what they see as the biggest challenges to sleep related practice growth, 81% replied medical billing/insurance.” 48% also reported “securing a medical diagnosis” as the second leading challenge. All too often, dental referrals for a sleep diagnosis result in a physician’s prescription for a CPAP or hypoglossal nerve surgery. As a result, sleep dentistry being practiced by about 1% of the general dentist population (2,000 American Academy of Dental Sleep Medicine certified out of 200,000+ dentists).
“I know that when I scan a closed bite for aligners or an oral appliance, I won’t have to worry about misalignment of the occlusal plane.”
– Dr. Mendy Ritchie, DDS, Braced Orthodontics in Marble Falls, Texas
Chair Time is Productivity.
New CAD/CAM techniques can take a closed-bite scan and digitally open the mouth. This process ensures no impact on the bite registration with skews of the midline or misalignment of the occlusal plane. Nor does it require special training for technicians. Closed-bite scans are how leading scanning manufacturers train dental technicians to scan for teeth aligners. The oral appliance market traditionally does open bite scans using a gauge to keep the teeth open during the scan. But For Dr. Mendy Ritchie, DDS of Braced Orthodontics in Marble Falls, Texas, the closed-bite scan helps her claw back valuable chair time. “I know that when I scan a closed bite for aligners or an oral appliance, I won’t have to worry about misalignment of the occlusal plane. No matter how practiced you are at using a gauge to create an open bite scan, the patient will invariably shift their jaw during the scan impacting the midline or occlusal plane. The usual result of this is a required adjustment on the finished product.” Dr. D’Alfonso agrees. “Refits and adjustments of improperly fitting devices take valuable chair time impacting the dentist and the entire staff.” Though closed bite scanning is not taught as a technique in most oral appliance design or continuing education courses, Slow Wave successfully uses it for all the products it makes. Another software innovation that complements closed-bite scanning is the use of artificial intelligence (AI) which is helping to streamline the design process of both aligners and oral appliances. The result is an OAT product that requires only one visit, fits perfectly the first time, does not require adjustment, reduces the liability of the dentist, and has a dramatically higher compliance rate.
Digital printing innovation is also advancing quickly. Formlabs 3D printers, with FDA cleared materials to produce dental products, could manufacture three oral appliances in a 5-hour print run in 2023. Today, the Formlabs Form4 can print the same three appliances in a quarter of the time without any loss of accuracy (1/100th of a mm), plus the design center can put the print job on a Formlabs printer anyplace in the world. It is just a matter of time before every dentist’s office is printing devices locally in office or at their local lab, regardless of where the design work is being accomplished.
These new FDA dual-cleared OAT products, innovations, technological advancements provide dentists and oral appliance suppliers with the market share growth opportunity that has eluded them thus far. The question now is whether the industry will take advantage of the opportunities it has been afforded. Will dentists quit looking at “sleep dentistry” as a forbidden fruit? Will bruxism devices finally help erode the “80% undiagnosed” talking point that has plagued the industry for decades? Will oral appliance therapy finally become a profitable mainstream product that every dentist prescribes for patients who walk in their doors?
Read more about the FDA dual clearance for the Slow Wave DS8 for obstructive sleep apnea and bruxism, here: https://dentalsleeppractice.com/industry-news/slow-wave-gets-fda-dual-clearance-on-the-slow-wave-ds8-for-obstructive-sleep-apnea-bruxism-marketed-as-brosa/.
- https://www.ama-assn.org/delivering-care/public-health/what-doctors-wish-patients-knew-about-sleep-apnea
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2727690/
- https://www.fortunebusinessinsights.com/industry-reports/sleep-apnea-devices-market-100708
- https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleid=52512
- https://www4.palmettogba.com/pdac_dmecs/searchProductClassificationResults.do?manufacturer=&codeDecision=K1027&productName=&modelNumber=&classification=&producttable_length=10
- Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of Obstructive sleep apnea in the general population. A systematic review. Sleep Med Rev 2017;34:70-81
- Maluly M, Andersen ML, Dal-Fabbro C, et al. Polysomnographic study of the prevalence of sleep bruxism in a population sample. J Dent Res 2013;92(7 suppl);97S-103S
- Manfredini D, winocur E, Guarda-Nardini L, Paesani D, Lobbezoo F. Epidemiology of bruxism in adults; A systemicreview of the literature. J Orofac Pain 2013; 27:99-110
- Hoysosa H, Yamaguchi T, Mikami S, et al. Relationship between sleep bruxism and sleep respiratory events in patients with obstructive sleep apnea syndrome. Sleep Breath 2014;18:837-844
- https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpcd/classification.cfm?id=213
- Manns A, D.D.S, Miralles R D.D.S, Cumsille F M.S, Vertical Dimension and EMG activity; Journal of Prosthetic Dentistry 1885;v53:243-247
 Wayne Wagner is the founder and CEO of Slow Wave, Inc., an expert in 3D engineering, and a long-time OSA sufferer.
Wayne Wagner is the founder and CEO of Slow Wave, Inc., an expert in 3D engineering, and a long-time OSA sufferer.