
This paper presents some personal thoughts, none of which should be taken as final pronouncements at this stage. My hope is to spark further thinking, open discussions and relevant research in the field, as well as collaborations with other healthcare professionals.
Currently, we are coping with major challenges in dental sleep medicine. We must be able to:
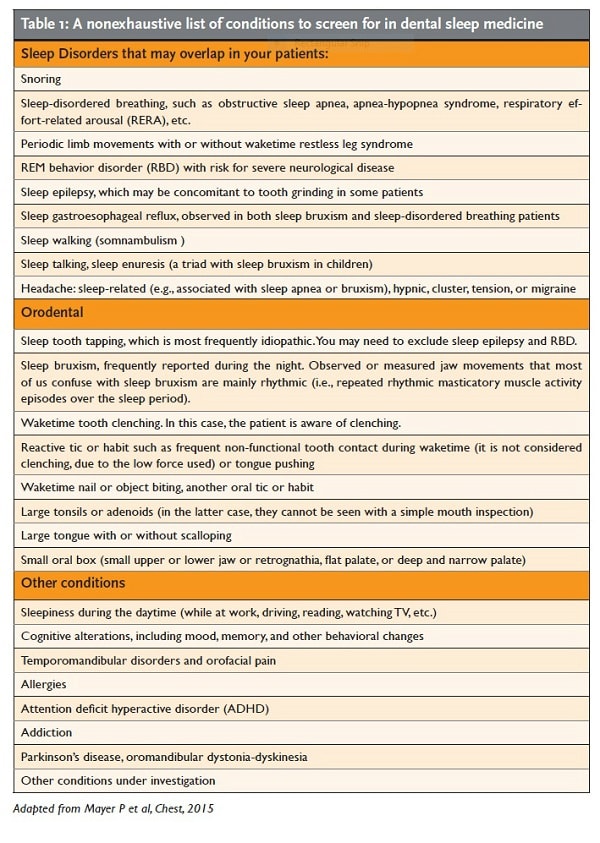
- be aware of the complexity of sleep- disordered breathing (SDB) in order to make accurate screening or differential diagnoses while taking into account the impact of comorbidities on management planning (see Table 1).
- understand the principles of precision medicine, a comprehensive, patient- centered approach.
- improve access to healthcare, from prevention and screening to diagnosis and treatment, in order to address this critical public health issue.
- balance information obtained from evidence-based medicine and dentistry with guidelines for daily clinical practice.
- translate sleep society guidelines into practice and develop efficient updating strategies for the fast-moving world of sleep medicine.
The prevalence of SDB has risen dramatically over the past two decades, from 14% to 55%, depending on the patient group, i.e., age and gender who are also critical factors (Peppard PE et al, Am J Epidemiol 2013). Prevention and early detection of SDB are critical due to the health consequences, which frequently begin in adolescence and peak in adulthood. These consequences include metabolic alterations (higher fasting insulin and, blood glucose plus insulin resistance) as well as higher risks for cardiovascular morbidity and mortality, and accidents due to sleepiness (Bhushan B et al, Int J Pediatr Otorhinolaryngol. 2015; Mukherjee S et al, American Journal of Respiratory and Critical Care Medicine, 2015; Peppard PE et al, Am J Epidemiol 2013). Fortunately, dental sleep medicine is making continuous advances in terms of knowledge and technology.
Unlike my usual publications, this is not a report of a randomized clinical trial, an experimental trial, or a systematic meta-analysis. My intention was to gather together some recurrent thoughts arising from lectures I have delivered to dental and medical practitioners in various parts of the world. These thoughts have been inspired by your comments and questions as well as my own reading and ongoing research. I have chosen to publish this paper in a nonacademic style journal in order to reach an audience of dentists in clinical practice.
The Challenges of Dental Sleep Medicine
The dentist’s role in sleep had been primarily for sleep bruxism management but about 30 years ago it strongly emerged for sleep disordered breathing with the development of a few oral appliances that were designed to reduce snoring and help preserve airway patency during sleep. Clinicians and scientists in dentistry and medicine began sharing their experiences, questions, and visions in an unprecedented way. Sleep medicine became a shining example of an integrated, interdisciplinary health domain. Of course, not everything is perfect, and much progress is needed before optimal dental care can be provided to the greatest number of SDB patients in need.
 Some clinicians continue to view dental sleep medicine as a jackpot field. More rationally, most of us realize that sleep medicine is a demanding domain of dentistry. Patients with sleep disorders have special needs and specific expectations that require a different approach than the usual dental-tooth-periodontal care.
Some clinicians continue to view dental sleep medicine as a jackpot field. More rationally, most of us realize that sleep medicine is a demanding domain of dentistry. Patients with sleep disorders have special needs and specific expectations that require a different approach than the usual dental-tooth-periodontal care.
In restorative dentistry, we focus on the immediate outcome. In other words, we fix problems that we can see in front of us. In contrast, SDB management requires us to shift this modus operandi to pursue a more long-term objective of patient wellbeing. In SDB, the patient is the central figure, and we inform and guide patients according to their expectations, beliefs, and medical and socioeconomic situations.
In sleep medicine, improved health appears to be better achieved by:
- active patient involvement in management, such as diet, exercise, and sleep hygiene
- high patient compliance in the use of mechanical tools such as oral appliances and continuous positive airway pressure (CPAP) or sleep position devices
- in some cases, physical or myofunctional therapy and psychology, such as cognitive behavioral therapy (CBT), or nerve stimulation to open the airway, or corrective surgery on the nose, maxillary, or upper airway.
So far, no medications have been recognized or approved as treatments for managing SDB, except for patients who are living at high altitudes.
Oral appliances, albeit considered the second choice after traditional CPAP devices for SDB management, have been suggested to be equally effective in the long term for reducing morbidity and mortality (Young D and Collop N, Curr Treat Options Neurolo 2014; Anandam A et al, Respirology 2013; Bratton DJ et al, JAMA 2015). However, this significant finding needs to be replicated to reassure those who remain skeptical about the benefits. As scientists, we are skeptical by nature and by training.
The fact that both oral appliances and CPAPs are mechanical methods that work by improving airway function during sleep raises questions in my mind. Are they not only the best but also the only effective methods for preventing or improving SDB, including the consequences for health? The future of these devices in sleep medicine remains an open question, and particularly when we consider the substantial burden of SDB in terms of health and medical costs. Simple cases need to be identified early in life (i.e., in children), and preventive actions need to be taken to avoid more extensive care in adults with high medical risks or those who hold decision-making positions requiring alertness and fully cognitive functioning, such as aircraft pilots, finance investors, surgeons and… politicians. Children with craniofacial syndromes such as Pierre Robin or with recurrent infections or metabolic syndromes should receive comprehensive early treatment (Tan HL et al, Sleep Med Review, 2015).
Moreover, the value of orthodontics and preventive or corrective surgery to treat obstructive sleep apnea in children is debatable. Most children with retrognathia and narrow palate appear to benefit from palatal expansion and improved airway. However, adenotonsillectomy is not a panacea for all children. It was recently shown to improve obstructive sleep apnea in only 25% of children, and in only 10% of obese cases (Koren D et al, CHEST, 2015). This finding suggests that certain phenotypes (i.e., physical and biochemical characteristics and their interactions with genetics and the environment) need to be identified to predict best outcomes in a given SDB population. This approach is called precision medicine (PM), as described below.
Precision medicine in dentistry: From a one-size-fits-all paradigm to an advanced decision-making process
In the last three decades, the dentistry field has introduced innovations that are at once amazing and polarizing. They include implants, aesthetic dentistry, 3D imaging, electronic aids, and restorative dentistry tools such as periodontal biomaterials. Gene and immune mediators for diagnosis and therapeutics are also gaining ground, and although it is generally recognized that they cannot resolve all issues, they have opened up promising avenues for future interventions.
It is well known that patients differ in terms of biological and environmental risk factors. Hence, more integrated and intelligent phenotyping (e.g., morphology, familial history, health status and life style, genetic and immune biomarkers) is needed to help estimate the probability that cluster risk and predicted success factors are associated with the highest outcome probability.
Wikipedia currently defines Precision Medicine (PM) according to the National Research Council’s vision, as follows: Precision Medicine refers to the tailoring of medical treatment to the individual characteristics of each patient. It does not literally mean the creation of drugs or medical devices that are unique to a patient, but rather the ability to classify individuals into subpopulations that differ in their susceptibility to a particular disease, in the biology and/or prognosis of those diseases they may develop, or in their response to a specific treatment. Preventive or therapeutic interventions can then be concentrated on those who will benefit, sparing expense and side effects for those who will not. Although the term ‘Personalized Medicine’ is also used to convey this meaning, that term is sometimes misinterpreted as implying that unique treatments can be designed for each individual (https://en.wikipedia.org/wiki/Precision_medicine).
Bearing this in mind, dentists may tend to do what they feel is best based on their own beliefs. However, beliefs and convictions are not supposed to be the modus operandi of medical professionals, and decision making in dentistry should not involve gambles. Healthcare decisions should be based on the best available evidence and assessments of the benefits and risks for the patient as well as the patient’s capacity. In this case, capacity refers to the patient’s medical condition, age, and socioeconomic situation. It does not mean the dentist’s capacity to say, “I think so,” or “I’m telling you!” In PM, a growing number of tools are available for use by doctors, not as disinterested technicians, but in order to make decisions in collaboration with the patient.
These tools include molecular diagnostics (currently under development; e.g., Nizankowska-Jedrzejczyk A et al, J Clin Sleep Med 2014; DeLuca Canto G et al, Sleep Medicine 2015), imaging (already used to improve airways, with brain imaging developments expected), and analytical software (see a preliminary model in Trenaman et al, Sleep Breathing, 2015). Algorithm-based analytical software will allow sharing information stored in large databanks to help fine-tune tools such as phenotyping for decision making. This approach also raises new ethical issues to consider, and patients’ consent will be required.
It is worth repeating here that the use of an apnea-hypopnea index (AHI) alone is insufficiently reliable or predictive of the health risks when selecting a treatment and assessing outcomes. This index should be used as a guide, and not a hard-and-fast rule. If assessments of hypoxia and autonomic activity along with the patient’s risk factors and medical and familial history are missing, we may find ourselves in a blurry situation of maybe yes, maybe no. I call this the gray area of patient assessment. Other challenges include too many false negatives in at-risk cases, too much variability in one-night recordings due to AHI fluctuations over time in mild cases, and lack of interest in the role of intermittent hypoxia on morbidity and mortality (Cairns A et al, J Clin Sleep Med, 2014; Punjabi NM et al, PLOS 2009; Punjabi NM, CHEST 2015).
We also need to gain a deeper understanding of phenotyping. We must be aware that certain psychosocial, anatomical, biological, and clinical risk factors are frequently clustered in certain population subgroups. This calls for more precise diagnostic and treatment decisions. For instance, a recent study on the benefits of PM found that patients with resistant hypertension showed a better response (i.e., reduced blood pressure) when treated with a CPAP within a cluster of patients having 3 (plasma) mRNA (Sanchez-de-la Torre, M et al., J Am Coll Cardiol, 2015).
Simple cases need to be identified early in life (i.e., in children), and preventive actions need to be taken to avoid more extensive care in adults with high medical risks.
Dentistry cannot progress without embracing modern biotechnologies that incorporate our clinical examination findings, are valid, and are accessible. If they are too costly, they will not be accessible. Furthermore, no machine can replace the dentist’s role in patient diagnosis, or the personal input required to manage treatments and to inform, reassure, comfort, provide relief to, and follow the patient. A sleep recording device can assist in the decision-making process and help guide management planning. We can look forward to more precise methods and tools in the future as well. Nevertheless, most methods and tools involve an inherent degree of uncertainty. Based on my experience, I expect from 5 to 30% false positives or negatives with any new approach. For example, a large population study on patients who underwent screening and portable recording suggests the need for personal attention to patients with a history of insomnia, stroke, and/or lung disease and with a low apnea-hypopnea index (Cairns A et al, J Clin Sleep Med, 2014). Although oral appliances are used in combination with more precise monitoring tools to manage SDB, the patient’s medical history and any new medical events should always be taken into consideration.
Evidence-based and Management Approaches
It is a no-brainer that dentists should select the best management approach. This could include screening, a physician’s diagnosis, or treatment with an oral appliance, CPAP, or surgery, always based on the strongest available evidence.
In the presence of SDB, recent guidelines recommend that sleep physicians should prescribe oral appliance therapy administered by a qualified dentist (Ramar K et al, J Cli Sleep Med, 2015). This recommendation is based on the literature and consensus between the physician and dentist. However, although consensus guidelines are great tools that help us share a common language and strategies, they are not completely free of bias, and their relevance may have a short shelf life as new evidence emerges. They are meant as guides, and not definitive prescriptions for SDB management. It is important to retain a critical stance in order to select the best treatment in a professional manner. It is where PM and evidence based medicine are coming important.
What is more, recent guidelines are silent on what to do when patients present other dental conditions or comorbidities. In the presence of sleep bruxism alone, that is, with no evidence of SDB or insomnia, we must assess the role of bruxism in pain or headache onset or recurrence, tooth damage, and quality of life. At that point, we may have to decide that a referral to a sleep physician is or is not necessary. But what if you suspect that SDB, sleep bruxism, insomnia, or tooth tapping (which is a sign of potential sleep-related epilepsy or RBD, a neurodegenerative condition)? Incidentally, the so-called interrelationship between sleep bruxism and SDB needs further corroboration, as concluded in a recent review (Mayer P et al, Chest 2015).
Although a randomized control trial design is the strongest method for initial assessment of the effectiveness and advantages of new treatments, we should remember that in the case of medications, most trials are run by private firms in the aim of obtaining government approval, and they do not necessarily address actual clinical settings. Before adopting a new clinical procedure, it is essential to get a physician’s professional opinion. We need to balance the freshness of a new discovery with common sense. When a medication or device gets approved, data are provided to convince healthcare decision makers that there is a reasonable efficacy–safety ratio for a given population. However, only time and real-world follow-up will confirm the appropriateness and effectiveness of new treatments. Effectiveness studies are also known as pragmatic studies: they “examine interventions under circumstances that more closely approach real-world practice, with more heterogeneous patient populations, less-standardized treatment protocols, and delivery in routine clinical settings” (Singal AG et al, Clinical and Translational Gastroenterology, 2014). These studies provide the most convincing evidence, but they take years to complete, and multicentre collaboration is needed to get enough subjects to participate for shorter periods. Should we wait years? Of course not! We have to decide on the best management strategy now, based on the patient’s condition, capacity (physical, psychological, and economic), and needs. I should remind you here that the government approval process for oral appliances differs from that for medications. It is much less rigorous. But again, we have to wait for long-term follow-up studies on effectiveness. Time is therefore a major limiting factor.
Comorbidities: Screening and Diagnosis
Many dentists are surprised to find out that we actually do not cure bruxism. Instead, we manage or reduce the consequences, including tooth damage, grinding sounds, pain, and headache. In this respect, it is similar to SDB management. However, patients frequently have comorbidities. So what should we do?
Some of your patients may have sleep breathing disorders that are associated with sleep bruxism or temporomandibular disorders (TMD). These may be coincidental, due to the patient’s age. This is known as interesting epidemiology. For example, children and teenagers tend to grind their teeth, middle-aged women have a higher probability of TMD, and older patients have higher probabilities of snoring and sleep apnea. We need to identify the patient’s phenotype (i.e., morphological, environmental, or genetic), comorbidities, and other risk factors. And we should keep in mind that no definitive causal associations have been found in the interactions between sleep breathing disorders and either TMD or bruxism. Do not fall for an attractive one-size-fits-all paradigm.
As dentists working in dental sleep medicine, we are responsible for being competent to provide a sound sleep medicine screening and, when indicated, differential diagnosis. We have the expertise to diagnose sleep bruxism, orofacial pain, and TMD. Physicians, for their part, can diagnose other conditions such as SDB, insomnia, RBD, the causes of sleepiness and cognitive alterations during daytime, unstable hypertension, unexplained headache, and so on. However, we should be able to recognize these conditions, screen for them, and request referrals for a final diagnosis. Table 1 presents a list of comorbidities that you may come across. Please note that this list is not exhaustive. For instance, it does not include all the craniofacial syndromes.
Each time you collect biological signals with these recording devices, you are responsible for ensuring that the data are carefully read and interpreted by an expert in the field.
Because not all physicians have expertise in sleep medicine, we are also responsible for referring patients to trained sleep physicians when there are potential risk factors (e.g., obesity, hypertension, sleepiness, retrognathia, mood and/or cognitive alterations, craniofacial syndrome). The screening tools that dentists use are generally based on history, dental and oral examinations, questionnaires (Epworth for sleepiness, or Stop-Bang for apnea), or a combination (exam and questionnaire) with or without type 3 (three–four-channel) or 4 (one-channel) recording devices. Type 3 recording devices are economical and easy to use at home to monitor sleep breathing and jaw or leg muscle activity when a breathing disorder, bruxism, or periodic limb movements are suspected. They include just a few channels (for breathing, muscle activity, oxygen, and sometimes brain activity) and use intelligent software to guide the examiner to make a diagnosis. Nevertheless, no machine is perfect, nor can it replace a human healthcare professional. The diagnosis falls within the doctor’s purview, and when SDB is at issue, the diagnosis should be made by a trained sleep physician. Type 4 devices use one muscle channel (leg or jaw) or finger oximetry recording. This is fine for first-line detection and for rapid, low-cost CPAP or oral appliance monitoring. However, oximetry alone cannot discriminate central from obstructive sleep apnea, nor can identify the apnea or hypopnea events occurring in the absence of oxygen desaturation. For atypical and moderate-to-severe cases (mainly if a medical comorbidity is present, or if sleepiness or cognitive alterations are reported), a full sleep laboratory or home polysomnography system (type 1 or 2) under medical supervision is the ideal tool for scoring and diagnosis. Moreover, each time you collect biological signals with these recording devices, you are responsible for ensuring that the data are carefully read and interpreted by an expert in the field.
There has been much debate recently about the need for more and better SDB diagnosis and management, presaging a move from the expert physician-only paradigm to a more open approach, including minimally sleep-trained family physicians and nonphysicians. This possible change, if it ever happens, should take place in an organized fashion so as to improve early prevention and care for simpler cases (Phillips B et al. Am J Respir Crit Care Med. 2015). Again, this does not mean a free-for-all. Instead, this calls for the development of professional collaborations. Solo performances are counter-indicated in sleep medicine.
To improve your expertise in patient screening, I suggest the following:
- Take formal continuing education courses in sleep medicine, and not just in dentistry, and attend medical sleep meetings to keep abreast of new developments.
- Join independent dental sleep academies.
- Join a study club where dentists, physicians, and other sleep-related professionals (psychologists, respiratory technicians, etc.) can share their experiences and ideas.
- Fine-tune your expertise in recognizing comorbidities.
- Move on from the traditional silo dentistry model and build a collaborative network. It will benefit both you and your patients in terms of health and quality of life.
N.B.: References cited and related abstract are available at: http://www.ncbi.nlm.nih.gov/pubmed/.