Dr. Suzanne R. Mericle and Jeff Wyscarver delve into how Dental Remote Patient Monitoring brings useful data about efficacy and compliance to a dental sleep practice
 by Suzanne Mericle, DMD, and Jeff Wyscarver, RPSGT
by Suzanne Mericle, DMD, and Jeff Wyscarver, RPSGT
❝When performance is measured, performance improves. When performance is measured and reported back, the rate of improvement accelerates.❞ – Karl Pearson
A poll of approximately 100 Board-Certified sleep physicians revealed that 68% believed CPAP works well. However, only 55% responded that Oral Appliance Therapy (OAT) works well.1 These physician biased perceptions result in PAP being prescribed more than ten times as often as OAT. Making these statistics even more surprising is if you describe oral appliance therapy and CPAP therapy to an undiagnosed apneic, 80% will select OAT. So why are 92% of prescriptions for OSA treatment for CPAP when 80% of patients prefer OAT?
I recently conducted an unofficial poll of 40 dental sleep medicine (DSM) practitioners and 15 of them reported, “ask the patient how they are doing” as their methodology to determine OAT efficacy. Subjective reporting as a standalone when assessing a sleep disorder is definitely negligent and potentially perilous.
Sleep centers commonly perform split night studies. An attended polysomnogram (PSG) is performed for the purpose of diagnosing OSA. Once sufficient evidence of OSA is recorded, the attending polysomnographic technician implements a trial of nasal PAP to determine the PAP pressure required to eliminate or greatly reduce the presence of disordered breathing. The second phase of titration comes in the form of PAP devices that auto-titrate. Internal “smart” technology determines the amount of PAP pressure required to normalize the airway on a continual basis. Many PAP systems delivered today are auto-titrating units which essentially circumvent the need for a specific PAP setting previously determined by a titration study.
One of the unexpected outcomes of the Covid-19 pandemic is that the titration portion of PSGs are not being performed out of an abundance of caution. Due to Covid-19 and concerns about the aerosol risk inherent to split night studies, many sleep centers have eliminated PAP titration and split night studies. They are only conducting diagnostic PSGs. Historically, omitting the titration process was unthinkable, but now it is the standard of care in many environments. The American Academy of Sleep Medicine (AASM) has stated “…when the possibility of increased risk to others exists, positive airway pressure delivered via mask should be based on a thorough risk-benefit analysis…”2 Let’s now discuss what happens once a patient is diagnosed with OSA and is either wearing an auto-PAP (APAP) or an oral appliance and how compliance and efficacy are monitored.
Comparing Apples to Aardvarks
I’ll paraphrase business management legend, Peter Drucker, “If you can’t measure it, you can’t improve it.” This is certainly true of sleep therapeutics. PAP efficacy is rather high, but patient compliance is relatively low.3 The inverse is somewhat true with OAT; high compliance but efficacy data is murkier.3 APAP units provide a variety of parameters such as a flow signal and hours per night used via technology built into the APAP devices (Fig.1). These data are transmitted to the durable medical equipment (DME) provider to document compliance and efficacy for each of the initial 90 nights. This compliance information is so essential that DME companies do not get reimbursed unless it is documented the patient is using the PAP device a minimum amount of time for the first 90 nights.4
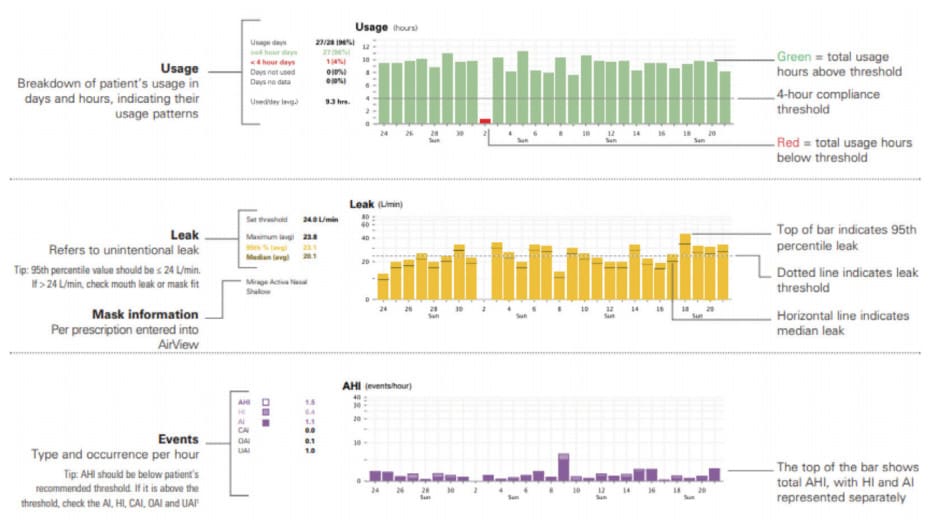
Dentists are not currently tasked with providing this information, and among many physicians, this is a common reproach leading to the dearth of OAT prescriptions. To measure OAT effectiveness post-treatment, Home Sleep Tests (HST) or pricey PSGs are usually performed for up to two nights. How does a clinician determine if the OAT titration process is efficacious over a period of multiple nights? And what about compliance? The differences between PAP and OAT as it relates to monitoring is like comparing apples to aardvarks.
The Current State of Affairs
The DSM profession has taken steps in the right direction. A growing number of dentists use an oximeter for multiple nights to gather measurable oxygen saturation information as an indicator of efficacy. This is helpful but still inadequate.
The DentiTRAC (Braebon) provides compliance data, but this device is not available for many appliances and doesn’t provide any efficacy information (Fig.2).

More useful data about efficacy and compliance over a longer period of time is needed. How can DSM clinicians assure our physician colleagues that a shared patient’s OAT is efficacious, and they are compliant for a significant window of time? Dental Remote Patient Monitoring (DRPM) is the answer.
What is DRPM?
Recent advancements in wearable technology have produced a great solution for dentists. Monitoring a patient’s titration for a few weeks, months, or longer provides several advantages and addresses the previously detailed shortcomings. Measuring sleep over many nights is achievable due to a new wearable technology – the CIRCUL. The CIRCUL indicates efficacy through SPO2, heart rate, and a 4 stage Sleep Quality histogram: wake, light sleep, deep sleep, and REM sleep (Fig. 3). We propose these parameters provide better data than APAP devices as they measure the actual patient’s response to therapy, as opposed to the performance of the equipment.
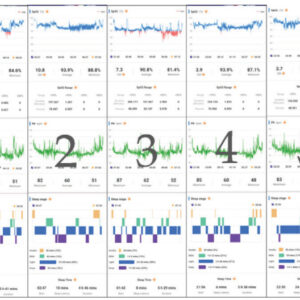
By presenting dentists with a trove of real-time efficacy information, the CIRCUL can improve OAT patient outcomes. This data sharing creates a compelling story that ensures patients are engaged in their therapy. This can create an element of gamification which has been demonstrated to improve health outcomes.5
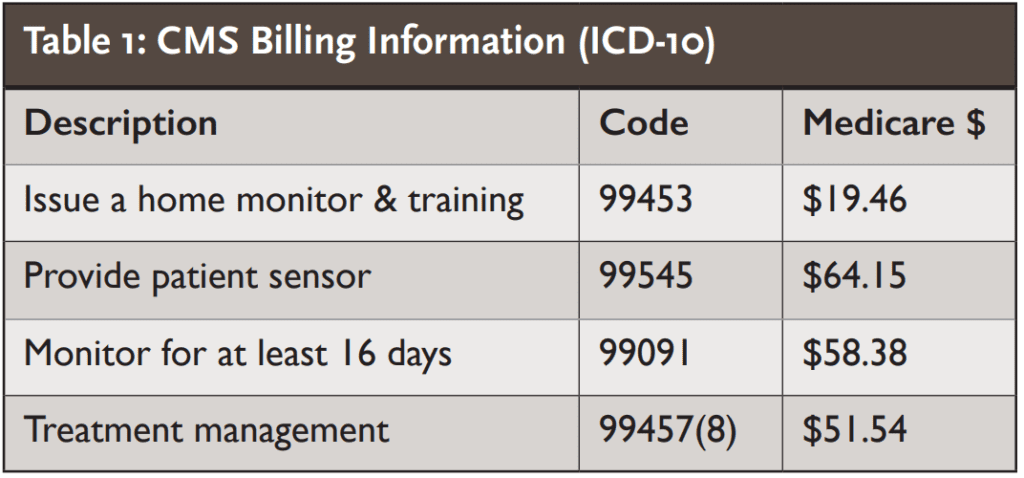
Implementing DRPM into a practice is simple, and there are 4 relevant billable codes, so we have a clinically sound improvement coupled with new reimbursement codes.
There are two business models available to achieve DRPM using the CIRCUL:
- The CIRCUL is available for less than $300. It can be dispensed and reused by the dentist similar to how oximeters are dispensed today. However, the CIRCUL can be used for longer stretches of time at half the cost of traditional oximetry with twice as much meaningful data
- Alternatively, the wearable can be sold directly to the patient.
According to CMS, there are multiple RPM codes available. Per CMS, you can bill for these codes if you are a “physician or other qualified healthcare professional.” (See Table 1.)
 Little discussion has been devoted to the distinction between PAP therapy and OAT as it relates to the titration process. This distinction is one of the primary reasons the medical community has been reluctant to adopt OAT as a preferred treatment modality. DRPM empowers you to monitor your patient’s treatment efficacy and compliance. This will lead to healthier patients actively engaged in their treatment and more physician referrals as they realize that we’re comparing apples to apples.
Little discussion has been devoted to the distinction between PAP therapy and OAT as it relates to the titration process. This distinction is one of the primary reasons the medical community has been reluctant to adopt OAT as a preferred treatment modality. DRPM empowers you to monitor your patient’s treatment efficacy and compliance. This will lead to healthier patients actively engaged in their treatment and more physician referrals as they realize that we’re comparing apples to apples.
Dental Remote Patient Monitoring and telemedicine are both practice game-changers. Find out how telemedicine opens doors to your practice here:
https://dentalsleeppractice.com/practice-management/game-changer/
- Grannick, L (2019). Sleep Physician OAT Prescription Trends. Fletcher Spaght International.
- “COVID-19: FAQs for Sleep Medicine Clinicians and Sleep Facilities: AASM.” American Academy of Sleep Medicine – Association for Sleep Clinicians and Researchers, American Academy of Sleep Medicine, 28 Aug. 2020, aasm.org/covid-19-resources/covid-19-faq/.
- Mandibular advancement splints and continuous positive airway pressure in patients with obstructive sleep apnoea: a randomized cross-over trial
- “Positive Airway Pressure (PAP) Devices – Clinician Frequently Asked Questions.” Noridian Medicare, Noridian Healthcare Solutions, Dec. 2008, med.noridianmedicare.com/documents/2230703/17635061/PAP+Devices+FAQs.
- Johnson, Daniel, et al. “Gamification for Health and Wellbeing: A Systematic Review of the Literature.” Internet Interventions, Elsevier, 2 Nov. 2016, ncbi.nlm.nih.gov/pmc/articles/PMC6096297/.