The FDA-cleared Slow Wave DS8 offers patient comfort and high efficacy. Read more about this next-generation custom 3D-printed oral appliance for sleep apnea and snoring.
 Slow Wave, Inc. manufactures the newly FDA-cleared and multi-patented Slow Wave DS8, a next generation oral appliance for the treatment of sleep apnea and snoring. The unique design of Slow Wave DS8 features a focus on incorporating a vertical opening of 6 mm to 8 mm that allows the mandible to move forward in a natural position rather than pulling the jaw forward. The result is both patient comfort and high efficacy. There is no need for a morning repositioner for patients using the Slow Wave DS8. This is also the first FDA-cleared oral appliance to be 3D printed on a Formlabs printer from a digital scan that provides accuracy to within 100th of a millimeter. This provides custom patient fit without the need for appliance adjustment. The Slow Wave DS8 has even been granted a unique billing code by Medicare/Medicaid due to its unique design features.
Slow Wave, Inc. manufactures the newly FDA-cleared and multi-patented Slow Wave DS8, a next generation oral appliance for the treatment of sleep apnea and snoring. The unique design of Slow Wave DS8 features a focus on incorporating a vertical opening of 6 mm to 8 mm that allows the mandible to move forward in a natural position rather than pulling the jaw forward. The result is both patient comfort and high efficacy. There is no need for a morning repositioner for patients using the Slow Wave DS8. This is also the first FDA-cleared oral appliance to be 3D printed on a Formlabs printer from a digital scan that provides accuracy to within 100th of a millimeter. This provides custom patient fit without the need for appliance adjustment. The Slow Wave DS8 has even been granted a unique billing code by Medicare/Medicaid due to its unique design features.

The Slow Wave market approach is to enable dentists to leverage what they already do — identify patients, perform scans, and provide patient follow-up. Slow Wave eliminates the need for dentists to take on tasks they don’t want, like referrals with sleep specialists, neurologists, or other medical professionals to get a medical diagnosis or perform medical billing services for an oral appliance. The Slow Wave network handles the sleep study, diagnosis, and medical billing.
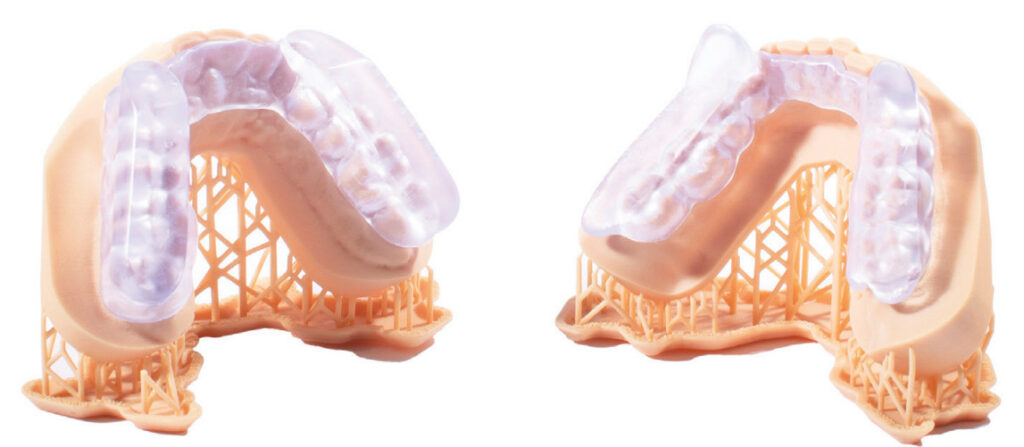
Because the Slow Wave DS8 is custom printed on a Formlabs printer, it can happen at the Slow Wave lab or right in the dentist’s office. It is possible for patients to be scanned in the morning and take home a custom-fitted appliance that solves their sleep apnea or snoring issues later that day! Slow Wave handles all of the product design and can print directly to the printer in the dental office. We even help with printer setup, supplies, and training for your dental office team.
Learn More
A new CMS code K1027, which became effective on October 1, opens the door for OSA oral appliances that follow an all-digital design and manufacturing path. Read more about the approval of the Slow Wave DS8 in “Slow Wave DS8 first OAT approved by CMS under HCPCS Code level II (K10270)” here: https://dentalsleeppractice.com/industry-news/new-oat-oral-appliance-therapy-medical-billing-code-issued-for-the-treatment-of/

