Randy Clare discusses Medicare-reimbursed appliances and how to obtain approvals for underserved patients.
 by Randy Clare
by Randy Clare
Medicare provides coverage for more than 100 million people. That’s nearly 1 in 3 Americans. When combined with Medicaid, coverage is extended to 125 million people. The number of people at risk of sleep disordered breathing is skewed much higher in these populations due to comorbid conditions and known risk factors.
This patient population is grossly under-treated with oral appliances for sleep apnea despite the undeniable need. Low reimbursement for oral appliance therapy is commonly cited as a contributing factor. This glaring gap is exacerbated by CMS’s requirement of pricey PDAC approved Herbst style custom appliances. It’s important to note that an increasing number of private insurers are adopting Medicare’s appliance design requirements, too.
It’s unfortunate but true. Cost is a factor. It is important that we negotiate with these programs in terms of economics and the size of the patient population. Only then can we transition from empty pronouncements to actionable quality-centric and cost-based solutions.
While both are administered by CMS, Medicare and Medicaid are very different programs.
Medicare is a national health insurance program that provides health insurance for Americans 65 years or older. Medicare also provides coverage for individuals with recognized disabilities such as renal disease and ALS.
Medicaid is a federal and state program that provides benefits for people with limited income and resources.
Medicaid is an extensive program available in every state. It covers nearly 20% of non-elderly Americans, providing coverage for low-income families, pregnant women, children, and people with disabilities.
CMS reports that Medicaid consumes over 28% of state budgets running a very close second to education which registered 29% based on a report of expenditures FY2017. That year total Medicaid expenditures amounted to ~$600 billion.
More than 1 million doctors, health care providers, and suppliers participate in CMS programs. While dentistry is a covered Medicaid service, coverage varies by state and is primarily focused on emergency care and improving children’s access to dental care. There are state oral action plans filed by over 25 states that are available for review.
Sleep Apnea Appliances for Medicare and Medicaid
CMS uses a standardized procedure coding system called Healthcare Common Procedure Coding System (HCPCS). Oral appliance therapy has been assigned the HCPCS code E0486.
For an MAD like Silent Nite to qualify for E0486, it must have premarket clearance by the FDA.
HCPCS code E0486 states, “…oral device/appliance used to reduce upper airway collapsibility, adjustable or non-adjustable, custom fabricated, includes fitting and adjustment.”
I am unaware of any lab-fabricated oral appliances on the market today that do not meet this definition.
However, for the device to be suitable for Medicare patients, it must also be approved by Pricing, Data Analysis & Coding (PDAC).
PDAC requirements are more stringent, detailed, and demanding. The related policy article clearly outlines the following:
“Code E0486 may only be used for custom fabricated mandibular advancement devices. To be coded as E0486, custom fabricated mandibular advancement devices must meet all of the criteria below:
- Have a fixed mechanical hinge (see below) at the sides, front or palate; and,
- Be able to protrude the individual beneficiary’s mandible beyond the front teeth when adjusted to maximum protrusion; and,
- Incorporate a mechanism that allows the mandible to be easily advanced by the beneficiary in increments of one millimeter or less; and,
- Retain the adjustment setting when removed from the mouth; and,
- Maintain the adjusted mouth position during sleep; and,
- Remain fixed in place during sleep so as to prevent dislodging the device; and,
- Require no return dental visits beyond the initial 90-day fitting and adjustment period to perform ongoing modification and adjustments in order to maintain effectiveness (see below)
A fixed hinge is defined as a mechanical joint, containing an inseparable pivot point. Interlocking flanges, tongue and groove mechanisms, hook and loop or hook and eye clasps.’
Most dental labs have responded to this requirement by making a Herbst style appliance with an expensive hinge accessory to satisfy the CMS standard. Many dentists rightfully complain that the lab fee for the appliance consumes most of the reimbursement.
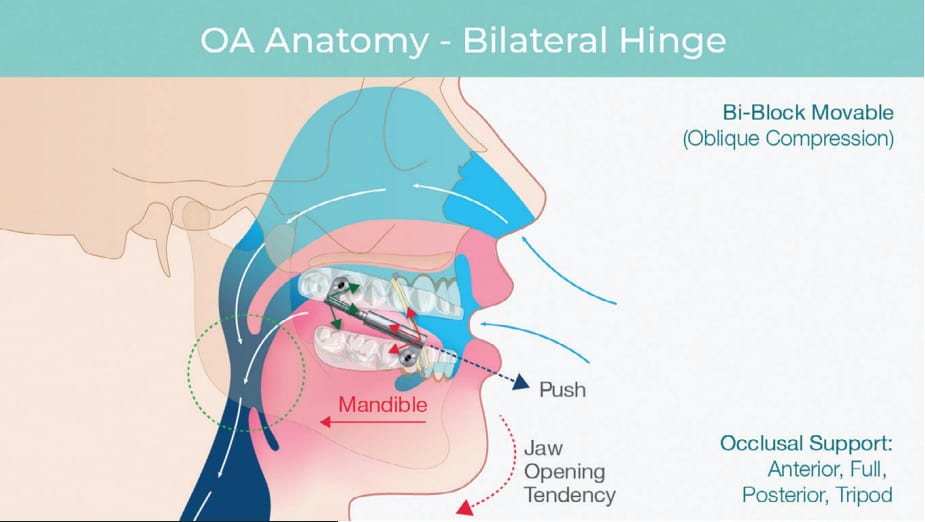
The Glidewell Hinge is Revolutionary
These issues created the demand and opportunity for the new Glidewell Hinge. Glidewell’s Research and Development team started with the basic principles that the final appliance needed to cost less than $250, had to satisfy the CMS criteria for PDAC approval, and it needed to build on the Silent Nite framework so that it is easy for dentists to deliver with materials found in a standard dental office.
The Glidewell Hinge is the most recent PDAC approved accessory for an MAD to qualify for E0486. Dentists can now order a Silent Nite with a Glidewell Hinge which is a Herbst style bilateral compression MAD for the treatment of OSA.

The Glidewell Hinge is a stainless-steel hinge mechanism that can be adjusted while in the patients mouth if necessary. Calibrated score marks on the Glidewell Hinge “arms” help dentists easily balance appliance titration. There are also additional features like the elastic hooks.
The Silent Nite with the Glidewell Hinge is a lab-processed, custom-fabricated PDAC approved device.
The Glidewell Hinge makes the Silent Nite a bilateral compression device which pushes the jaw into a therapeutic protrusive position. To prevent undesirable mouth opening during sleep which can result in dry mouth or suboptimal treatment, lugs on the upper tray allow the addition of elastic bands to support the jaw in a closed position encouraging lip seal and quiet nasal breathing.
Conclusion
The need for an effective treatment modality for Medicare and Medicaid demographics is clear. These patients deserve sleep apnea treatment. For many, this would be oral appliance therapy under the care of a licensed dentist.
The decision to provide OAT in a dental practice involves consideration of multiple factors. The scope of this article does not allow for an exhaustive review of these factors, but patient demographics, profitability, and practice protocols should all be considered when making the decision to provide oral appliances.
Medicare allowed amounts for oral appliance therapy range from ~$1,000 to about $1,900. Mandibular advancement devices, especially those for Medicare patients, must incorporate a few non-negotiable attributes for successful treatment and desirable profitability. The appliance must be affordable, PDAC approved, and fabricated without any special tools.
Success in the business of dental sleep medicine requires low appliance cost, standardized clinical workflow protocols, and building case volume. The Silent Nite with the Glidewell Hinge costs less than $250. This is a lab-processed, custom-fabricated PDAC approved device that requires 4 days in lab and includes all the standard warranties for which Glidewell is known.
This is clearly a strategy that will improve access to oral appliance therapy for patient populations that are severely underserved while improving the profit margins for practices that provide this much needed care.
To read more on Medicare-reimbursed appliances for OSA, read Dr. Ken Berley’s article on Medicare rules and guidelines here: https://dentalsleeppractice.com/need-know-medicare-guidelines-oral-appliances-osa-friend-foe-part-4/


