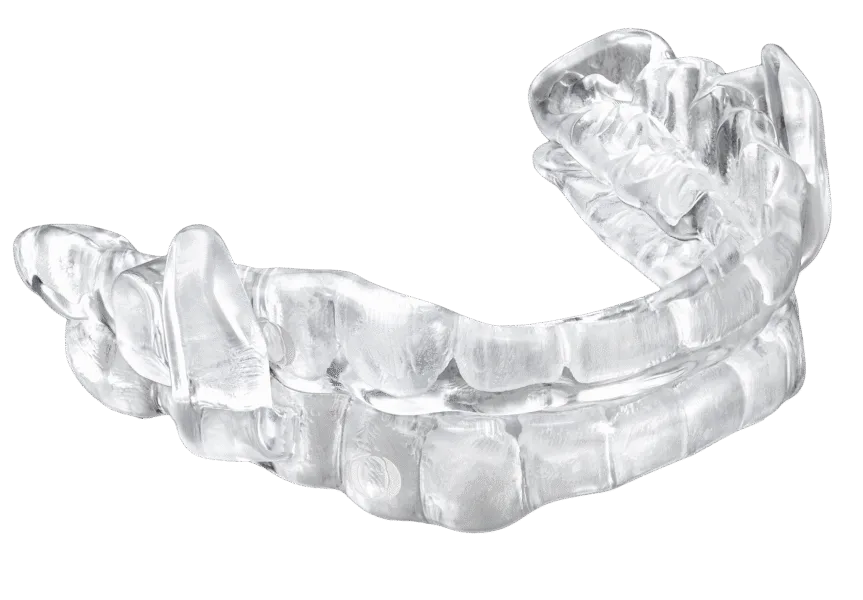
EVO Guided is the first oral device bioengineered to dilate the oropharynx and the velopharynx
SAN FRANCISCO, June 5, 2025 – ProSomnus Sleep Technologies (the “Company”), the leading non-CPAP Obstructive Sleep Apnea (OSA) therapy®, announces EVO Guided. EVO Guided is a new precision, custom oral device for the treatment of Obstructive Sleep Apnea (“OSA”). FDA cleared, patented and covered by most medical insurances, EVO Guided is the first oral device bioengineered to dilate the oropharynx and the velopharynx for optimal therapeutic success.
The Insight: The Limitations of Traditional Mandibular Advancement
Watanabe et al achieved 94% success in treating severe OSA patients with manual jaw thrust maneuvers1, demonstrating the tremendous potential for mandibular repositioning. However, observed success rates drop below 65% when traditional, semi-custom oral appliances are used to protrude the mandible2. Why the differences in success rates between manual jaw thrust maneuvers and advancement with traditional oral appliances? This velopharynx is the collapse location for 94% of events1, while the oropharynx is involved in just 56%3. Research demonstrates that protruding the mandible with traditional oral appliances has direct effects on oropharyngeal dilation, but only indirect effects on velopharyngeal dilation.
The Solution: Purpose Driven Engineering
EVO Guided is engineered to encourage the oral mechanics linked with elevated levels of treatment success, while minimizing, if not avoiding, counterproductive mechanics such as inaccurate mandibular repositioning and stabilization, posterior tongue posture, and forcing larger than necessary separation of the upper and lower teeth. EVO Guided has a 95-degree guided prescription post titration mechanism that guides the mouth closed and stabilizes the mandible within microns of the target therapeutic position. It features lingual-less maxillary and mandibular dental overlays that encourage a beneficial, anterior tongue posture to minimize deformation and collapse of the ventral wall of the velopharynx.
“We designed EVO Guided with enhanced efficacy in mind, prioritizing 3-dimensional precision mandibular positioning and maximizing tongue space,” commented Sung Kim, CTO. “EVO Guided is our continued commitment to advanced, patient-centric Oral Appliance Therapy for improved OSA treatment.”
Unlike traditional oral appliances that feature prefabricated components and modifications, EVO Guided is 100% personalized based on records from each individual patient’s oral structures without prefabricated components or modifications. In clinical studies, ProSomnus devices have demonstrated non-inferiority to CPAP4; superior symptom alleviation5; patient preference over traditional oral appliances6; and mitigation of dental side effects7.
“The ProSomnus EVO Guided device has a true custom, anatomical design that maximizes efficacy by guiding mandibular repositioning and guiding the tongue anteriorly while maintaining mouth closure, noted Edward T. Sall, MD, DDS, MBA. As both a physician and dentist treating patients, I believe it should be considered as the new ‘gold standard’ for Oral Appliance Therapy in the treatment of OSA.”
Early Clinical Feedback on EVO Guided is Encouraging:
“ProSomnus continues to innovate. What’s their motivation to improve a device that is already ‘best of class’? Ah yes…better compliance, better results. We will never get 100%, but the EVO Guided device moves the needle in the right direction and provides a comfortable lingual-less design,” stated Kent Smith, D-ABDSM, ASBA.
“The new ProSomnus EVO Guided device is super easy for insertion; offers a perfect fit every time and patients have reported it to be extremely comfortable. Given the very low profile and lingual-less design, it’s becoming a preferred device option for our GoTo Sleep Centers,” commented Stacey C. Layman DDS, D-ABDSM.
“The lingual-less design, refined post structure, and precision engineering of EVO Guided provide greater tongue space and comfort—key factors in enhancing efficacy and patient outcomes,” commented Michael Gelb, DDS, MS.
“ProSomnus EVO devices fit seamlessly with zero adjustments. The new EVO Guided device, featuring an additional 5 degrees of post taper, eliminates the need for elastics to manage jaw drop—its enhanced post angulation provides ideal support. Existing EVO users who have upgraded to EVO Guided appreciate the increased tongue space due to reduced appliance bulk, allowing for greater comfort. With less material interference, less protrusion may be necessary, enabling the tongue to achieve its natural, unobstructed position more efficiently,” stated David E. Federici, DMD.
"EVO Guided is now my go-to oral device. Having made over 1,500 devices, I see the impact of its lingual-less design, 95-degree lower guided post, and improved lip closure—offering patients more tongue space, reduced protrusion, and greater comfort. The seamless chairside delivery makes treatment effortless, and patient feedback has been overwhelmingly positive, with more predictable results,” mentioned John A. Carollo, DMD, FAASM, D-ABDSM
"The recently introduced ProSomnus EVO Guided device addresses several patient concerns prevalent in previous models. This device provides enhanced tongue space, increased resistance to mouth opening, and a compact design that minimizes bulk within the buccal vestibules. My patients have expressed their satisfaction with the comfort of their new device. It is my new favorite appliance,” noted Dana Blalock, DDS.
"I just had my first night with my new ProSomnus EVO Guided device, and it was the best sleep I've had in years. After 8 years of CPAP, followed by 10 years of using every type of device, this is by far the most comfortable device I’ve ever used. There was absolutely no interference with my tongue—just the natural feel of my teeth and palate. It was as if I had nothing in my mouth at all. Everyone needs an EVO Guided device!” stated Alan Blanton, DDS, MS, D. ABDSM.
“ProSomnus EVO Guided represents a major step forward in the evolution of Oral Appliance Therapy—intelligently simplifying the treatment of OSA without compromising efficacy or precision. Designed with the clinician and patient in mind, the device leverages years of scientific research, bioengineering expertise, and countless anatomical data points from intra-oral precision scans to create what I would call ‘intelligent simplicity’. EVO Guided is more than a device; it’s a solution. It empowers more dentists to treat with confidence, streamlines device selection, and brings us closer to true personalization in the era of precision medicine. It’s a breakthrough that reflects the deep understanding of OSA pathophysiology and the practical realities of clinical care. I believe EVO Guided will have a major impact on expanding access to better health for more patients,” noted Shouresh Charkhandeh, DDS, BMedSc.
"ProSomnus has pushed the bar higher with its new EVO Guided device. Reduced thickness and minimal encroachment of the tongue space, the ability to be a velopharynx dilator, and the elimination of needing elastics all lead me to now choose the EVO Guided as my number one choice for my patients,” commented Ben Utterback, DDS, D.ABDSM
EVO Guided is engineered to precisely reposition and stabilize the mandible. It features a lingual-less splint design to create more room for the tongue and allow a natural contact pressure between the tongue and the lingual dental anatomy.
1Watanabe et al. (2002). Contribution of body habitus and craniofacial characteristics to segmental closing pressures of the passive pharynx in patients with sleep-disordered breathing. AJRCCM; 165:260-265.
2Leonard A. Liptak et al. (2025). Different Oral Appliance Designs Demonstrate Different Rates of Efficacy for the Treatment of Obstructive Sleep Apnea: A Review Article. Bioengineering; 2025, 12, 210
3Watanabe et al. (2002). Contribution of body habitus and craniofacial characteristics to segmental closing pressures of the passive pharynx in patients with sleep-disordered breathing. AJRCCM; 165:260-265.
4Dieltjens et al. (2004). Overall clinical effectiveness of oral appliance therapy as a first-line treatment option compared with continuous positive airway pressure in patients with moderate to severe obstructive sleep apnea: The FLOSAT study. JDSM; 2024 AADSM annual meeting abstracts and case reports.
5Loerger et al. (2024). Mandibular advancement vs combined airway and positional therapy for snoring. JAMA Otolaryngol Head Neck Surg; 150(7):572-579.
6Ryser et al. (2024). Soldier preference in mandibular advancement devices in patients who brux. J Dent Oral Epidemiol; 4(2):1-7.
7Vranjes et al. (2019). Assessment of potential tooth movement and bite changes with a hard-acrylic sleep appliance: A 2-year clinical study. JDSM; 6(2).
About ProSomnus Sleep Technologies
ProSomnus Sleep Technologies is the leading non-CPAP therapy for the treatment of Obstructive Sleep Apnea, a serious medical disease affecting over 1 billion people worldwide, that is associated with comorbidities including heart failure, stroke, hypertension, morbid obesity, and type 2 diabetes. ProSomnus intraoral medical devices are engineered to precisely track the treatment plan and anatomy for each patient. Non-invasive, patient preferred and easy to use, ProSomnus devices have demonstrated excellent efficacy, safety, adherence, and overall outcomes in a growing body of clinical investigations. ProSomnus precision intraoral devices are FDA-cleared, patented, and covered by commercial medical insurance, Medicare, TRICARE and many Government-sponsored healthcare plans around the world, representing over 200 million covered lives. To learn more, visit www.ProSomnus.com.