CE Expiration Date: May 1, 2027
CEU (Continuing Education Unit):2 Credit(s)
AGD Code: 730
Educational Aims
This self-instructional course for dentists aims to present the FAIREST-6 tool. All professionals assessing children for possible sleep-related breathing disorders, poor growth of the craniofacial respiratory complex (CFRC), and potential speech and swallowing dysfunctions need a tool to make screening more objective. This article presents the most well-developed tool for this process, the FAIREST-6. It also highlights the role of the Speech-Language Pathologist as a key player in identifying these at-risk children.
Expected Outcomes
Dental Sleep Practice subscribers can answer the CE questions online to earn 2 hours of CE from reading the article. Correctly answering the questions will demonstrate the reader can:
- Easily document several objective measurements of the CFRC.
- Be able to communicate the importance of these measures with families.
- Feel confident in collaborative care with other health care providers in this field.
Ann Blau and Kaitlyn Shrum explore the role of speech-language pathologists and their integral role in screening children’s sleep-related issues.
 by Ann Blau, MS, CCC-SLP, CMT® and Kaitlyn Shrum, MA, CCC-SLP, QOM, CMT®
by Ann Blau, MS, CCC-SLP, CMT® and Kaitlyn Shrum, MA, CCC-SLP, QOM, CMT®
Sleep is one of the greatest public health challenges of the 21st century. The American Academy of Pediatrics estimates that sleep problems affect 25-50% of children and 40% percent of adolescents. At least 12-14% of children have some form of sleep-disordered breathing (SDB) and roughly one to five percent have a formal diagnosis of obstructive sleep apnea (OSA). SDB and OSA can adversely impact children’s speech, language, and cognitive development as well as their craniofacial and upper airway growth.
Since speech-language pathologists (SLPs) are often involved early in the assessment and treatment of communication and swallowing difficulties in the pediatric population, they are uniquely positioned to screen for sleep problems in these children. SLPs can incorporate The Functional Airway Evaluation Screening Tool (FAIREST-6) as part of their comprehensive speech and swallowing assessments to identify at-risk children and make appropriate and timely referrals when needed.
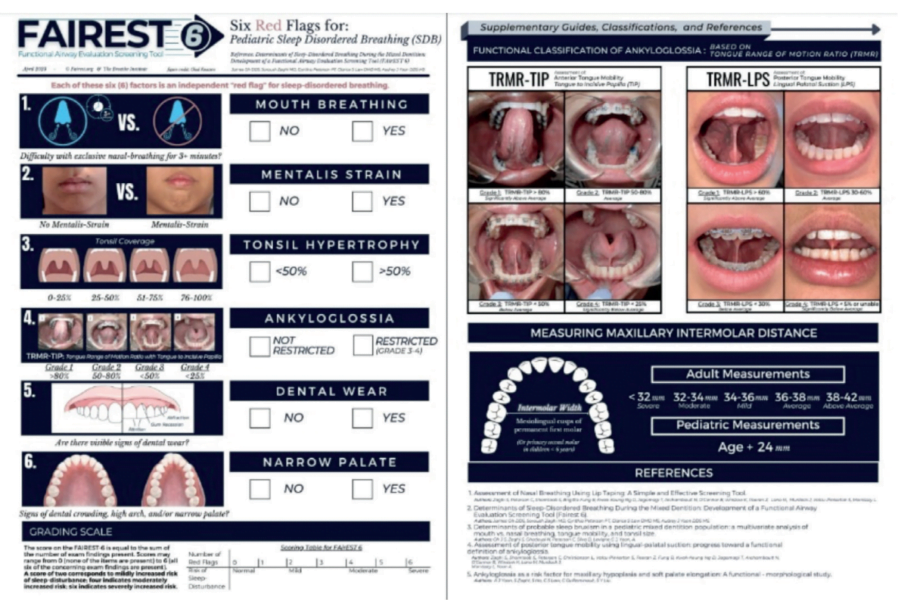
About the FAIREST-6
The Functional Airway Evaluation Screening Tool (FAIREST-6) is a concise and validated clinical airway screening tool that was developed to identify pediatric dental patients at risk for sleep-disordered breathing (SDB). It is comprised of six clinical determinants, each of which have been found to be independent red flags for SDB in children during the mixed dentition (the stage where both primary and permanent teeth are present). These include mouth breathing, mentalis strain, tonsil hypertrophy, ankyloglossia, dental wear, and a narrow palate.
The FAIREST-6 can be used by dentists, physicians, and allied health professionals to screen for pediatric SDB, aiding in early diagnosis and intervention in pediatric SDB. As SLPs, this is an easy-to-administer screening tool that can be used in a variety of settings to identify at-risk pediatric patients on our caseloads. Although the FAIREST-6 was initially validated on children between the ages of 6 and 12, there exists a potential for extended application to children above the age of 2.5- to 3-years-old, since the signs of pediatric SDB can be present from a young age and early identification is critical. While the FAIREST-6 does have some validity in older cohorts as well, there are other assessment tools that can be used that are more specific to those populations.
Visit https://www.fairest.org/ to download a copy of the FAIREST-6.
Each of the six determinants on the FAIREST-6 screening tool is an independent red flag for SDB, and a sign of orofacial myofunctional impairment.
Administration of the FAIREST-6
Each of the six red flags on the FAIREST-6 are discussed in detail below:
- Mouth Breathing refers to difficulty breathing through the nose with the lips sealed for >3 minutes.
Potential methods of assessment include:
- having the patient hold water in the mouth with the lips sealed for 3 minutes
- placing a tongue depressor between gently closed lips while the patient breathes through the nose for 3 minutes
- having the patient keep their lips closed while breathing through the nose for 3 minutes
If (c) is not possible, the lips may be gently sealed with MicroPore paper tape* while the patient breathes through the nose for three minutes. This protocol was implemented in the Zaghi et al. (2020) cross-sectional, multi-center cohort study. Among the 663 study participants between the ages of 3 and 83, those who were unable to breathe exclusively through the nose for 3 minutes while the lips were taped were found to have a significantly increased likelihood of mouth breathing while awake and asleep.
*Disclaimer: MicroPore paper tape in the pediatric population specifically should be utilized with extreme caution under the direct supervision of a knowledgeable healthcare provider and should be removed immediately at the first sign of respiratory distress.
If the patient cannot comply for the full three minutes, referral to an otolaryngologist for further assessment of upper airway patency is advised.
According to Warnier et al. (2023), the most efficient way for SLPs to assess mouth breathing in preschool children is via observation of the child at rest (i.e., time spent with an open/closed mouth and position of the tongue/lips). The child’s breathing pattern while chewing (open/closed mouth) and after swallowing (open/closed mouth just after swallowing) can provide additional clinically relevant data.
Characteristic features of mouth breathers include: lips open or parted at rest (instead of resting gently together), a shortened upper lip (showing the upper front teeth), a protruding or flaccid lower lip, an elongated face, venous pooling or dark circles under the eyes, narrow nostrils, weak cheek muscles, a high palate, narrowing of the upper jaw and malocclusion (misalignment of the teeth), altered head posture, dry lips and mouth, swollen and red gums that bleed easily. Additionally, mouth breathing patients may report symptoms such as snoring and open mouth while sleeping, chronic sinus and ear infections and colds, and chronic halitosis. During sleep, mouth breathing decreases intra oral pH which can lead to greater risk of tooth erosion, tooth decay, and increased tooth sensitivity. Chronic mouth breathing in growing children is associated with palatal growth restriction, alterations in craniofacial development, low tongue tone, reduced tongue strength, chewing inefficiency, sleep-disordered breathing, and increased risk for obstructive-sleep apnea later in life. The most important factors in ensuring adequate craniofacial and airway development in children are exclusive nasal breathing and proper oral rest posture.
- Mentalis Strain refers to lip incompetence characterized as moderate-severe straining of the chin muscle upon mouth closure.
At rest, the lips should remain closed with a relaxed mentalis. When there is a struggle to bring or keep the lips together, a strain or dimpling in the chin is seen. Mentalis strain is an extra-oral indicator of altered facial growth. It can be accompanied by a gummy smile due to a long, vertical face, a retrognathic (recessed) mandible, or underdevelopment of both the maxilla and the mandible. Mentalis strain can be indicative of oral incompetence, lip ties, weakness in the lips and skeletal discrepancies or changes.
- Tonsil Hypertrophy refers to enlarged tonsils that occupy >50% of the oropharyngeal width.
Enlarged tonsils can make the upper airway smaller, increase resistance to nasal breathing and (potentially) worsen SDB. The tonsils can be seen by asking the patient to open their mouth widely and depressing the tongue with a tongue depressor to better visualize the tonsils at the back of the throat. Tonsillar tissue is hypertrophic if it occupies more than 50% of the oropharynx upon opening. Tonsil size can be assessed using the Brodsky tonsillar grading scale. Brodsky Grade 3 (tonsils occupying 51-75% of the oropharynx) and Grade 4 (tonsils occupying greater than 75% of the oropharynx) are predictive of SDB. Since the peak period of tonsillar growth is between the ages of two and eight, that is the time when the lymphoid tissue is disproportionately large in relation to the craniofacial profile, which can be a risk factor for SDB. Tonsillar hypertrophy is associated with mouth breathing and a retrognathic mandible, which diminishes retroglossal airway space. Together with adenoid hypertrophy, it is the most common risk factor for obstructive sleep apnea (OSA) in children, as the resulting upper airway stenosis can obstruct airflow, thereby contributing to OSA development.
- Ankyloglossia, also known as tongue tie, refers to a congenital condition that impairs tongue movement due to a restrictive lingual frenulum.
A tongue tie restricts tongue mobility when the lingual frenulum, the midline fold of fascia and overlying mucosa that lies between the ventral surface of the tongue and the floor of the mouth, is unusually short, thick, or tight. Ankyloglossia interferes with normal tongue function by preventing the tongue from resting along the palate in its entirety, thereby affecting proper development of the craniofacial structures. Alterations of the lingual frenulum may contribute to orofacial myofunctional disorders, speech (e.g., lisps) and swallowing impairments (a tongue thrust or poor swallowing pattern) and even SDB. Ankyloglossia has also been associated with underdevelopment of the maxilla (or narrowing of the maxillary arch) and elongation of the soft palate, resulting in an anatomically smaller upper airway with increased risk of collapsibility.
A functional classification of restricted tongue mobility is the tongue range of motion ratio (TRMR), an independent measure of tongue mobility. Objective measurements of tongue mobility using the TRMR are taken with the tongue tip extended towards the incisive papilla (TIP) and with the tongue body held against the palate in lingual-palatal suction (LPS). The incisive papilla is a cartilaginous landmark on the hard palate that is posterior to the upper front teeth and anterior to the first palatal ridge. The TIP functional measurement is obtained by instructing the patient to lift the tip of the tongue behind the upper front teeth and opening the mouth as widely as comfortably possible without discomfort or pain. The TRMR-TIP serves as a measure of the variation in functional mobility of the anterior one-third (tongue tip and apex) of the tongue. It is calculated as the ratio between maximum mouth opening with vertical tongue extension to the incisive papilla (TIP) and maximum comfortable mouth opening capacity. Measurements are rated according to the following grading scale: Grade 1 = >80% (significantly above average), Grade 2 = 50-80% (average), Grade 3 = < 50% (below average), and Grade 4 = < 25% (significantly below average). TRMR-TIP Grades 3 and 4 are considered independent red flags for SDB.
The LPS functional measurement is obtained by instructing the patient to lift and suction the entire tongue to the palate (as if about to make a click sound) and opening the mouth as widely as possible without discomfort or pain. The TRMR-LPS serves as a measure of the variation in functional mobility of the posterior one-third (body) of the tongue. It is calculated as the ratio between maximum mouth opening with the tongue in lingual-palatal suction (LPS) and maximum comfortable mouth opening capacity. Measurements are rated according to the following grading scale: Grade 1 = > 60% (significantly above average), Grade 2 = 30-60% (average), Grade 3 = < 30% (below average), and Grade 4 = < 5% or unable (significantly below average). TRMR-LPS Grades 3 and 4 are considered independent red flags for SDB.
Overall, TRMR-TIP of less than 50% and TRMR-LPS of less than 30% are considered representative of moderately restricted anterior and posterior tongue mobility, respectively. It is also important to consider the influence of other clinical factors such as tension and compensation patterns when calculating these measurements.
An alternative functional classification system to the TRMR is the Kotlow free tongue measurement, an independent measure of tongue length and mobility. It is measured in millimeters as the distance from the tip of the tongue to the superior attachment of the lingual frenulum along the ventral tongue surface. Less than 16 mm is considered a clinically unacceptable value of free tongue movement and is classified as ankyloglossia.
- Dental Wear refers to irreversible moderate-severe deterioration of dental hard tissue resulting in dentin exposure by a mechanical or chemical process rather than tooth decay.
Two etiological factors implicated individually or synergistically (Nota et al., 2022) in dental wear are bruxism, a mechanical process, and gastroesophageal reflux disease (GERD), a chemical process.
Bruxism is defined as a repetitive masticatory muscle activity characterized by clenching or grinding of the teeth and/or by bracing or thrusting of the mandible. Sleep bruxism (or bruxism that occurs during sleep) is considered a major contributor to observable dental wear. It is thought that frequent arousals during sleep may signal activation of the jaw muscles to compensate for a restricted airway. In doing so, the lower jaw slides forward, opening the collapsed airway. A consequence of the protrusive sliding movement of the lower jaw against the upper jaw is friction on the teeth, which can lead to loss of tooth structure, cracked teeth and even tooth loss. Dental wear is considered a significant risk factor for SDB if dentin, the middle layer underneath the enamel of the tooth, can be seen.
GERD is a condition resulting in troublesome symptoms and/or complications due to retrograde flow of acidic stomach contents (digestive juices or food and fluid) into the esophagus. Sleep-related GERD in children is considered a risk factor for dental wear because gastric acid can displace saliva from tooth surfaces, resulting in dental erosion and demineralization. GERD is common in pediatric patients with digestive problems and obstructive sleep apnea.
- Narrow Palate refers to maxillary constriction which cannot accommodate the tongue at rest due to inadequate space.
To achieve proper growth and development of the maxilla, the tongue must reside in the palate, its physiological resting position. The constant pressure generated by the tongue resting in the roof of the mouth stimulates forward and lateral jaw growth as well as the development of the nasomaxillary complex and upper airway. When the tongue posture is low (such as in mouth breathing), the maxillary arch is often restricted with a V-shaped arch, high palatal vault and increased nasal airway resistance (since the same structure that forms the roof of the oral cavity serves as the floor of the nasal cavity). Dental crowding (a discrepancy between tooth size and jaw size), other malocclusions (misalignments of the teeth) such as an open bite (when the upper and lower teeth do not come in contact when biting down) or crossbite (when the upper teeth fit inside the lower teeth while biting down), a history of dental extractions due to limited space and increased facial height (an abnormally long inferior third of the face) can all be the result of an underdeveloped airway. A narrow maxilla leads to increased nasal resistance which promotes mouth breathing and subsequently SDB.
There are several different ways to measure palatal restriction. The Supplementary Guide of the FAIREST-6 provides an up-to-date and reliable method of assessing for maxillary transverse deficiency. In children, what constitutes ‘narrow’ can be referenced to the Bogue Index, which uses the algorithm of “chronological age + 24” to indicate sufficient development of the palatal arch. This indexed algorithm is based upon Dr. E.A. Bogue’s analysis of intermolar arch width in a large population of children under six years of age from the late 19th century through the early 20th century. Dr. Bogue postulated that in the absence of an intermolar distance of 28 mm between the mesiolingual cusps (the anterior cusps situated on the lingual side of the mandibular molar teeth) of the primary second molars in children under 6 years of age, and the permanent first molars in children above the age of 6, maxillary transverse deficiency was present.
In adults, an intermolar distance of less than 32 mm between the mesiolingual cusps of the first permanent molars is transverse deficient, which is comorbid with an SDB.
[Maxillary arch width can be estimated by assessing whether a cotton roll fits between the first permanent molars. A standard cotton roll is generally 37 mm long. A tool that can be used to measure arch length more accurately (in children or adults) is a dental caliper.]
Scoring of the FAIREST-6
The score on the FAIREST-6 is equal to the sum of the number of red-flag items present. Scores may range from 0 (none of the items are present) to 6 (all six of the concerning items are present). A score of 2 is associated with mildly increased risk of sleep-disturbance, a score of 4 is associated with moderately increased risk, and a score of 6 is associated with severely increased risk. Those with scores indicative of greater risk of SDB should be referred for a more comprehensive evaluation.
Each of the six determinants on the FAIREST-6 screening tool is an independent red flag for SDB, and a sign of orofacial myofunctional impairment.
The Role of the SLP in Routine Screenings for SDB
A recent publication in the ASHA Leader outlines a guide for the role of the SLP in children’s airway health. The article states, “SLPs can play a critical role in the prevention of, screening for, and interdisciplinary collaboration to diagnose SDB and related airway problems. We are able to intervene before significant problems take hold and become bigger issues for children, families, schools and society. By getting to the cause of the symptoms we see, we can help improve treatment outcomes, while ensuring that all our young clients live, learn and sleep with optimal airway function, 24/7” (Archambault 2018).
SLPs can identify signs and symptoms of SDB during their comprehensive speech, language, and swallowing evaluations via oral mechanism examination (including an assessment of structure and function), questionnaires, and a thorough parent/caregiver interview. They can screen for tongue space, tongue ties and mouth breathing habits, and refer to other medical professionals for further assessment of the upper airway. They can also observe behavioral signs of a possible SDB, such as excessive fidgeting, difficulty attending to tasks and emotional outbursts. SLPs may utilize information from standardized questionnaires and an in-depth intake to construct a comprehensive profile of the child’s sleep patterns, nighttime symptoms, and daytime functioning.
In addition to identification, SLPs can provide education to families regarding the possible impact of SDB on speech, language and swallowing. The SLP works closely with other health care professionals, including otolaryngologists, pediatricians, sleep specialists, allergists, etc. and can help develop a collaborative team to ensure the most comprehensive plan of care.
SLPs can therefore play a critical role in disease prevention in the children they serve by identifying those at highest risk for sleep disorders and other airway-related conditions via routine screening. Furthermore, since orofacial myofunctional disorders are prevalent in children displaying symptoms of SDB, we can also diagnose and treat concomitant orofacial myofunctional disorders to normalize orofacial resting posture and muscle function, which will in turn promote the development of a healthy airway.
Similar content was also presented as an iPoster at the American Speech-Language-Hearing Association Annual Convention, November 16-18, 2023, Boston, MA.
Acknowledgment: We would like to express our gratitude to Dr. Soroush Zaghi and his colleagues for granting us permission to share the latest version of the FAIREST-6 airway screening tool.
Speech language pathologists are uniquely qualified to help in the treatment of pediatric sleep disordered breathing. Read Sharon Moore’s article, “Face Facts: Function that Builds the Airway,” at https://dentalsleeppractice.com/face-facts-function-that-builds-the-airway/
References
- Alexander, N., Boota, A., Hooks, K., & White, J. R. (2019). Rapid maxillary expansion and adenotonsillectomy in 9-year-old twins with pediatric obstructive sleep apnea syndrome: An interdisciplinary effort. Journal of Osteopathic Medicine, 119(2), 126–134. https://doi.org/10.7556/jaoa.2019.019
- Alhazmi, W. (2022). Mouth breathing and speech disorders: A multidisciplinary evaluation based on the etiology. Journal of Pharmacy And Bioallied Sciences, 14(5), 911. https://doi.org/10.4103/jpbs.jpbs_235_22
- Archambault, N. (2018). Healthy Breathing, ’Round the Clock: Problems with airway functioning during sleep can hurt children’s health. And SLPs, alongside other professionals, are on the front lines of identification and intervention. The ASHA Leader, 23(2), 48–54. https://doi.org/10.1044/leader.FTR1.23022018.48
- Becker, S., Brizuela, M., & Mendez, M. D. (2023). Ankyloglossia(Tongue-tie). In StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK482295/
- Bhargava, S. (2011). Diagnosis and management of common sleep problems in children. Pediatrics In Review, 32(3), 91–99. https://doi.org/10.1542/pir.32.3.91
- Bogue, E. A. (1915). Judgment in orthodontics. International Journal of Orthodontia, 1(7), 354–356. https://doi.org/10.1016/S1072-3471(15)80060-1
- Bonuck, K., Battino, R., Barresi, I., & McGrath, K. (2021). Sleep problem screening of young children by speech-language pathologists: A mixed-methods feasibility study. Autism & Developmental Language Impairments, 6, 23969415211035066. https://doi.org/10.1177/23969415211035066
- Bonuck, K., Freeman, K., Chervin, R. D., & Xu, L. (2012). Sleep-disordered breathing in a population-based cohort: Behavioral outcomes at 4 and 7 years. Pediatrics, 129(4), e857–e865. https://doi.org/10.1542/peds.2011-1402
- Blunden, S., Lushington, K., Kennedy, D., Martin, J., & Dawson, D. (2000). Behavior and neurocognitive performance in children aged 5-10 years who snore compared to controls. Journal of clinical and experimental neuropsychology, 22(5), 554–568. https://doi.org/10.1076/1380-3395(200010)22:5;1-9;FT554
- Bozzini, M. F., & Di Francesco, R. C. (2016). Managing obstructive sleep apnoea in children: the role of craniofacial morphology. Clinics (Sao Paulo, Brazil), 71(11), 664–666. https://doi.org/10.6061/clinics/2016(11)08
- Brennan, L. C., Kirkham, F. J., & Gavlak, J. C. (2020). Sleep-disordered breathing and comorbidities: role of the upper airway and craniofacial skeleton. Nature and science of sleep, 12, 907–936. https://doi.org/10.2147/NSS.S146608
- Brzęcka, D., Garbacz, M., Micał, M., Zych, B., & Lewandowski, B. (2019). Diagnosis, classification and management of ankyloglossia including its influence on breastfeeding. Developmental period medicine, 23(1), 79–87. https://doi.org/10.34763/devperiodmed.20192301.7985
- Bulanda, S., Ilczuk-Rypuła, D., Nitecka-Buchta, A., Nowak, Z., Baron, S., & Postek-Stefańska, L. (2021). Sleep Bruxism in Children: Etiology, Diagnosis, and Treatment-A Literature Review. International journal of environmental research and public health, 18(18), 9544. https://doi.org/10.3390/ijerph18189544
- Byrd, R. S., Weitzman, M., Lanphear, N. E., & Auinger, P. (1996). Bed-wetting in US children: epidemiology and related behavior problems. Pediatrics, 98(3 Pt 1), 414–419.
- Carter, K. A., Hathaway, N. E., & Lettieri, C. F. (2014). Common sleep disorders in children. American Family Physician, 89(5), 368–377.
- Chervin, R. D., Archbold, K. H., Dillon, J. E., Panahi, P., Pituch, K. J., Dahl, R. E., & Guilleminault, C. (2002). Inattention, hyperactivity, and symptoms of sleep-disordered breathing. PEDIATRICS, 109(3), 449–456. https://doi.org/10.1542/peds.109.3.449
- Corrêa, C. D. C., Cavalheiro, M. G., Maximino, L. P., & Weber, S. A. T. (2017). Obstructive sleep apnea and oral language disorders. Brazilian Journal of Otorhinolaryngology, 83(1), 98–104. https://doi.org/10.1016/j.bjorl.2016.01.017
- Crabtree, V. M., Varni, J. W., & Gozal, D. (2004). Health-related quality of life and depressive symptoms in children with suspected sleep-disordered breathing. Sleep, 27(6), 1131–1138. https://doi.org/10.1093/sleep/27.6.1131
- Ebert, C. S., & Drake, A. F. (2004). The impact of sleep-disordered breathing on cognition and behavior in children: A review and meta-synthesis of the literature. Otolaryngology–Head and Neck Surgery, 131(6), 814–826. https://doi.org/10.1016/j.otohns.2004.09.017
- Gottlieb, D. J., Chase, C., Vezina, R. M., Heeren, T. C., Corwin, M. J., Auerbach, S. H., Weese-Mayer, D. E., & Lesko, S. M. (2004). Sleep-disordered breathing symptoms are associated with poorer cognitive function in 5-year-old children. The Journal of pediatrics, 145(4), 458–464. https://doi.org/10.1016/j.jpeds.2004.05.039
- Gozal, D., & Pope, D. W. (2001). Snoring during early childhood and academic performance at ages thirteen to fourteen years. Pediatrics, 107(6), 1394–1399. https://doi.org/10.1542/peds.107.6.1394
- Guilleminault, C., Huang, Y. S., Monteyrol, P. J., Sato, R., Quo, S., & Lin, C. H. (2013). Critical role of myofascial reeducation in pediatric sleep-disordered breathing. Sleep Medicine, 14(6), 518–525. https://doi.org/10.1016/j.sleep.2013.01.013
- Harding, R., Haszard, J. J., Schaughency, E., Drummond, B., & Galland, B. (2020). Parent report of children’s sleep disordered breathing symptoms and limited academic progress in reading, writing, and math. Sleep medicine, 65, 105–112. https://doi.org/10.1016/j.sleep.2019.07.018
- Harding, R., Schaughency, E., Haszard, J. J., Gill, A. I., Luo, R., Lobb, C., Dawes, P., & Galland, B. (2021). Sleep-related breathing problem trajectories across early childhood and academic achievement-related performance at age eight. Frontiers in Psychology, 12, 661156. https://doi.org/10.3389/fpsyg.2021.661156
- Heit, T., Tablizo, B. J., Salud, M., Mo, F., Kang, M., Tablizo, M. A., & Witmans, M. (2022). Craniofacial Sleep Medicine: The Important Role of Dental Providers in Detecting and Treating Sleep Disordered Breathing in Children. Children (Basel, Switzerland), 9(7), 1057. https://doi.org/10.3390/children9071057
- Helwany, M., & Rathee, M. (2023). Anatomy, head and neck, palate. In StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK557817/
- Hoang, D. A., Le, V. N. T., Nguyen, T. M., & Jagomägi, T. (2023). Orofacial dysfunction screening examinations in children with sleep-disordered breathing symptoms. The Journal of clinical pediatric dentistry, 47(4), 25–34. https://doi.org/10.22514/jocpd.2023.032
- Honaker, S. M., Gozal, D., Bennett, J., Capdevila, O. S., & Spruyt, K. (2009a). Sleep-disordered breathing and verbal skills in school-aged community children. Developmental Neuropsychology, 34(5), 588–600. https://doi.org/10.1080/87565640903133582
- Honaker, S. M., Gozal, D., Bennett, J., Capdevila, O. S., & Spruyt, K. (2009b). Sleep-disordered breathing and verbal skills in school-aged community children. Developmental Neuropsychology, 34(5), 588–600. https://doi.org/10.1080/87565640903133582
- Huynh, N. T., Morton, P. D., Rompré, P. H., Papadakis, A., & Remise, C. (2011). Associations between sleep-disordered breathing symptoms and facial and dental morphometry, assessed with screening examinations. American journal of orthodontics and dentofacial orthopedics : official publication of the American Association of Orthodontists, its constituent societies, and the American Board of Orthodontics, 140(6), 762–770. https://doi.org/10.1016/j.ajodo.2011.03.023
- Isaiah, A., Ernst, T., Cloak, C. C., Clark, D. B., & Chang, L. (2021). Associations between frontal lobe structure, parent-reported obstructive sleep disordered breathing and childhood behavior in the ABCD dataset. Nature Communications, 12(1), 2205. https://doi.org/10.1038/s41467-021-22534-0
- Isono, S., Shimada, A., Utsugi, M., Konno, A., & Nishino, T. (1998). Comparison of static mechanical properties of the passive pharynx between normal children and children with sleep-disordered breathing. American journal of respiratory and critical care medicine, 157(4 Pt 1), 1204–1212.
- Kaditis, A. G., Alonso Alvarez, M. L., Boudewyns, A., Alexopoulos, E. I., Ersu, R., Joosten, K., Larramona, H., Miano, S., Narang, I., Trang, H., Tsaoussoglou, M., Vandenbussche, N., Villa, M. P., Van Waardenburg, D., Weber, S., & Verhulst, S. (2016). Obstructive sleep disordered breathing in 2- to 18-year-old children: Diagnosis and management. European Respiratory Journal, 47(1), 69–94. https://doi.org/10.1183/13993003.00385-2015
- Kazakova, T., Danoff, R., Esteva, I., & Shchurin, A. (2023). Gastro-esophageal reflux disease in primary care practice: A narrative review. Annals of Esophagus, 6, 25–25. https://doi.org/10.21037/aoe-21-62
- Kumar, D. S., Valenzuela, D., Kozak, F. K., Ludemann, J. P., Moxham, J. P., Lea, J., & Chadha, N. K. (2014). The reliability of clinical tonsil size grading in children. JAMA otolaryngology– head & neck surgery, 140(11), 1034–1037. https://doi.org/10.1001/jamaoto.2014.2338
- Lam, D. J., Jensen, C. C., Mueller, B. A., Starr, J. R., Cunningham, M. L., & Weaver, E. M. (2010). Pediatric sleep apnea and craniofacial anomalies: a population-based case-control study. The Laryngoscope, 120(10), 2098–2105. https://doi.org/10.1002/lary.21093
- Lau, E. Y., Choi, E. W., Lai, E. S., Lau, K. N., Au, C. T., Yung, W. H., & Li, A. M. (2015). Working memory impairment and its associated sleep-related respiratory parameters in children with obstructive sleep apnea. Sleep medicine, 16(9), 1109–1115. https://doi.org/10.1016/j.sleep.2015.04.025
- Lin, L., Zhao, T., Qin, D., Hua, F., & He, H. (2022). The impact of mouth breathing on dentofacial development: A concise review. Frontiers in Public Health, 10, 929165. https://doi.org/10.3389/fpubh.2022.929165
- Lobbezoo, F., Ahlberg, J., Raphael, K. G., Wetselaar, P., Glaros, A. G., Kato, T., Santiago, V., Winocur, E., De Laat, A., De Leeuw, R., Koyano, K., Lavigne, G. J., Svensson, P., & Manfredini, D. (2018). International consensus on the assessment of bruxism: Report of a work in progress. Journal of oral rehabilitation, 45(11), 837–844. https://doi.org/10.1111/joor.12663
- Luo, R., Galland, B. C., Gill, A. I., Dawes, P., & Schaughency, E. (2018). Habitual snoring at age 3 years: Links with parent-rated remembering in daily life and academic achievement at age 7 years. Journal of Developmental & Behavioral Pediatrics, 39(2), 144–153. https://doi.org/10.1097/DBP.0000000000000524
- Luo, R., Harding, R., Galland, B., Sellbom, M., Gill, A., & Schaughency, E. (2019). Relations between risk for sleep-disordered breathing, cognitive and executive functioning, and academic performance in six-year-olds. Early Education and Development, 30(7), 947–970. https://doi.org/10.1080/10409289.2019.1593075
- Marino, A., Malagnino, I., Ranieri, R., Villa, M. P., & Malagola, C. (2009). Craniofacial morphology in preschool children with obstructive sleep apnoea syndrome. European journal of paediatric dentistry, 10(4), 181–184.
- Menzies, B., Teng, A., Burns, M., & Lah, S. (2022). Neurocognitive outcomes of children with sleep disordered breathing: A systematic review with meta-analysis. Sleep Medicine Reviews, 63, 101629. https://doi.org/10.1016/j.smrv.2022.101629
- Mills, N., Geddes, D. T., Amirapu, S., & Mirjalili, S. A. (2020). Understanding the lingual frenulum: Histological structure, tissue composition, and implications for tongue tie surgery. International Journal of Otolaryngology, 2020, 1820978. https://doi.org/10.1155/2020/1820978
- Moeller, J. L., Paskay, L. C., & Gelb, M. L. (2014). Myofunctional therapy. Sleep Medicine Clinics, 9(2), 235–243. https://doi.org/10.1016/j.jsmc.2014.03.002
- Mohammed, D., Park, V., Bogaardt, H., & Docking, K. (2021). The impact of childhood obstructive sleep apnea on speech and oral language development: A systematic review. Sleep Medicine, 81, 144–153. https://doi.org/10.1016/j.sleep.2021.02.015
- Nota, A., Pittari, L., Paggi, M., Abati, S., & Tecco, S. (2022). Correlation between Bruxism and Gastroesophageal Reflux Disorder and Their Effects on Tooth Wear. A Systematic Review. Journal of Clinical Medicine, 11(4), 1107. https://doi.org/10.3390/jcm11041107
- O’Brien, L. M., Mervis, C. B., Holbrook, C. R., Bruner, J. L., Klaus, C. J., Rutherford, J., Raffield, T. J., & Gozal, D. (2004). Neurobehavioral implications of habitual snoring in children. Pediatrics, 114(1), 44–49. https://doi.org/10.1542/peds.114.1.44
- Oh, J. S., Zaghi, S., Peterson, C., Law, C. S., Silva, D., & Yoon, A. J. (2021). Determinants of sleep-disordered breathing during the mixed dentition: Development of a functional airway evaluation screening tool(FAIREST-6). Pediatric Dentistry, 43(4), 262–272.
- Owens, J. A., Spirito, A., McGUINN, M., & Nobile, C. (2000). Sleep habits and sleep disturbance in elementary school-aged children: Journal of Developmental & Behavioral Pediatrics, 21(1), 27–36. https://doi.org/10.1097/00004703-200002000-00005
- Pacheco, M. C., Fiorott, B. S., Finck, N. S., & Araújo, M. T. (2015). Craniofacial changes and symptoms of sleep-disordered breathing in healthy children. Dental press journal of orthodontics, 20(3), 80–87. https://doi.org/10.1590/2176-9451.20.3.080-087.oar
- Ranjitkar, S., Kaidonis, J. A., & Smales, R. J. (2012). Gastroesophageal reflux disease and tooth erosion. International Journal of Dentistry, 2012, 1–10. https://doi.org/10.1155/2012/479850
- Shirke, S. R., & Katre, A. N. (2023). Association of Sleep-Disordered Breathing and Developing Malocclusion in Children: A Cross-Sectional Study. Cureus, 15(6), e39813. https://doi.org/10.7759/cureus.39813
- Singh, H., Chandwani, B., & Finkelman, M. (2011). Sleep Bruxism Related Tooth Wear as a Clinical Marker for Obstructive Sleep Apnea/Hypopnea Syndrome in Children [Master’s thesis, Tufts University School of Dental Medicine]. https://dl.tufts.edu/concern/pdfs/rn301c544
- Svingos, A., Greif, S., Bailey, B., & Heaton, S. (2018). The Relationship Between Sleep and Cognition in Children Referred for Neuropsychological Evaluation: A Latent Modeling Approach. Children (Basel, Switzerland), 5(3), 33. https://doi.org/10.3390/children5030033
- Tamkin J. (2020). Impact of airway dysfunction on dental health. Bioinformation, 16(1), 26–29. https://doi.org/10.6026/97320630016026
- Thomas, W. (1966). A statistical assessment of tooth sizes, arrangement and arch form obtained from dental casts preparatory to the development of computer programming of malocclusions. Master’s Theses. https://ecommons.luc.edu/luc_theses/2068
- Urschitz, M. S., Eitner, S., Guenther, A., Eggebrecht, E., Wolff, J., Urschitz-Duprat, P. M., Schlaud, M., & Poets, C. F. (2004). Habitual snoring, intermittent hypoxia, and impaired behavior in primary school children. Pediatrics, 114(4), 1041–1048. https://doi.org/10.1542/peds.2003-1145-L
- Wang, R., Zhu, Y., Chen, C., Han, Y., & Zhou, H. (2022). Tooth Wear and Tribological Investigations in Dentistry. Applied bionics and biomechanics, 2022, 2861197. https://doi.org/10.1155/2022/2861197
- Warnier, M., Piron, L., Morsomme, D., & Maillart, C. (2023). Assessment of mouth breathing by Speech-Language Pathologists: an international Delphi consensus. CoDAS, 35(3), e20220065. https://doi.org/10.1590/2317-1782/20232022065
- Yoon, A., Zaghi, S., Weitzman, R., Ha, S., Law, C. S., Guilleminault, C., & Liu, S. Y. C. (2017). Toward a functional definition of ankyloglossia: validating current grading scales for lingual frenulum length and tongue mobility in 1052 subjects. Sleep & breathing = Schlaf & Atmung, 21(3), 767–775. https://doi.org/10.1007/s11325-016-1452-7
- Zaghi, S., Peterson, C., Shamtoob, S., Fung, B., Kwok-keung Ng, D., Jagomagi, T., Archambault, N., O’Connor, B., Winslow, K., Peeran, Z., Lano, M., Murdock, J., Valcu-Pinkerton, S., & Morrissey, L. (2020). Assessment of nasal breathing using lip taping: A simple and effective screening tool. International Journal of Otorhinolaryngology, 6(1), 10. https://doi.org/10.11648/j.ijo.20200601.13
- Zaghi, S., Shamtoob, S., Peterson, C., Christianson, L., Valcu-Pinkerton, S., Peeran, Z., Fung, B., Kwok-Keung Ng, D., Jagomagi, T., Archambault, N., O’Connor, B., Winslow, K., Lano, M., Murdock, J., Morrissey, L., & Yoon, A. (2021). Assessment of posterior tongue mobility using lingual-palatal suction: Progress towards a functional definition of ankyloglossia. Journal of oral rehabilitation, 48(6), 692–700. https://doi.org/10.1111/joor.13144
- Xiao, L., Su, S., Liang, J., Jiang, Y., Shu, Y., & Ding, L. (2022). Analysis of the Risk Factors Associated With Obstructive Sleep Apnea Syndrome in Chinese Children. Frontiers in pediatrics, 10, 900216. https://doi.org/10.3389/fped.2022.900216
- Zhang, W., & Si, L. Y. (2012). Obstructive sleep apnea syndrome (OSAS) and hypertension: pathogenic mechanisms and possible therapeutic approaches. Upsala journal of medical sciences, 117(4), 370–382. https://doi.org/10.3109/03009734.2012.707253
- Zettergren-Wijk, L., Forsberg, C. M., & Linder-Aronson, S. (2006). Changes in dentofacial morphology after adeno-/tonsillectomy in young children with obstructive sleep apnoea–a 5-year follow-up study. European journal of orthodontics, 28(4), 319–326. https://doi.org/10.1093/ejo/cji119
- Ziliotto, K. N., dos Santos, M. F., Monteiro, V. G., Pradella-Hallinan, M., Moreira, G. A., Pereira, L. D., Weckx, L. L., Fujita, R. R., & Pizarro, G. U. (2006). Auditory processing assessment in children with obstructive sleep apnea syndrome. Brazilian journal of otorhinolaryngology, 72(3), 321–327. https://doi.org/10.1016/s1808-8694(15)30963-0
