The “Gold standard of OAT” — we all strive to achieve greater efficiency, exceptional patient comfort, and compliance. In his article, Dr. Mark Murphy examines the value of certain characteristics of OAT.
by Mark T. Murphy, DDS, D.ABDSM
Earning physicians’ trust in OAT requires that we utilize device designs that demonstrate better efficacy, are within the designated precision tolerances, and have exceptional patient comfort and compliance. We could call that the ‘gold standard’ of OAT.
A recent survey of prominent Dental Sleep Medicine clinicians exhibited a keen understanding of this. Device Fit, Size, Durability and Biocompatibility, the attributes most closely aligned with preferences of sleep physicians, all ranked significantly higher than attributes that are less aligned with the preferences of sleep physicians such as Ease of Delivery.1 We would all prefer the easiest delivery, but most would NOT be willing to trade Fit, Size, Durability or Biocompatibility for it. Secondarily, yet very important, is that precision milled devices like those from ProSomnus® Sleep Technologies, have fewer adjustments at delivery and at follow up visits. That saves time – chair time and patient time. If we want to earn physicians prescriptions for OAT instead of CPAP, we must evolve from the artisanal handmade varietals to the newer precision designed OAT device alternatives. Let us examine the risk and tradeoff values for each of these characteristics.
Table of Contents
Fit
Fit does not mean ease of insertion. Rather it refers to the precision approximation of the retainer-like platform. When the fit is excellent, teeth do not move.2 Prevention of this side effect by design is predictable today with the precision milled hard PMMA platforms. Nylon and soft lined OAT devices, even if milled or printed, do not provide the retainer-like fit that prevents unwanted movement.3,4
Fit also refers to how well the device manufacturing replicates the prescribed starting position. A recent comparative series demonstrated variability across most manufacturing that exceeded the standard AADSM guidelines for device design (Figure 1).
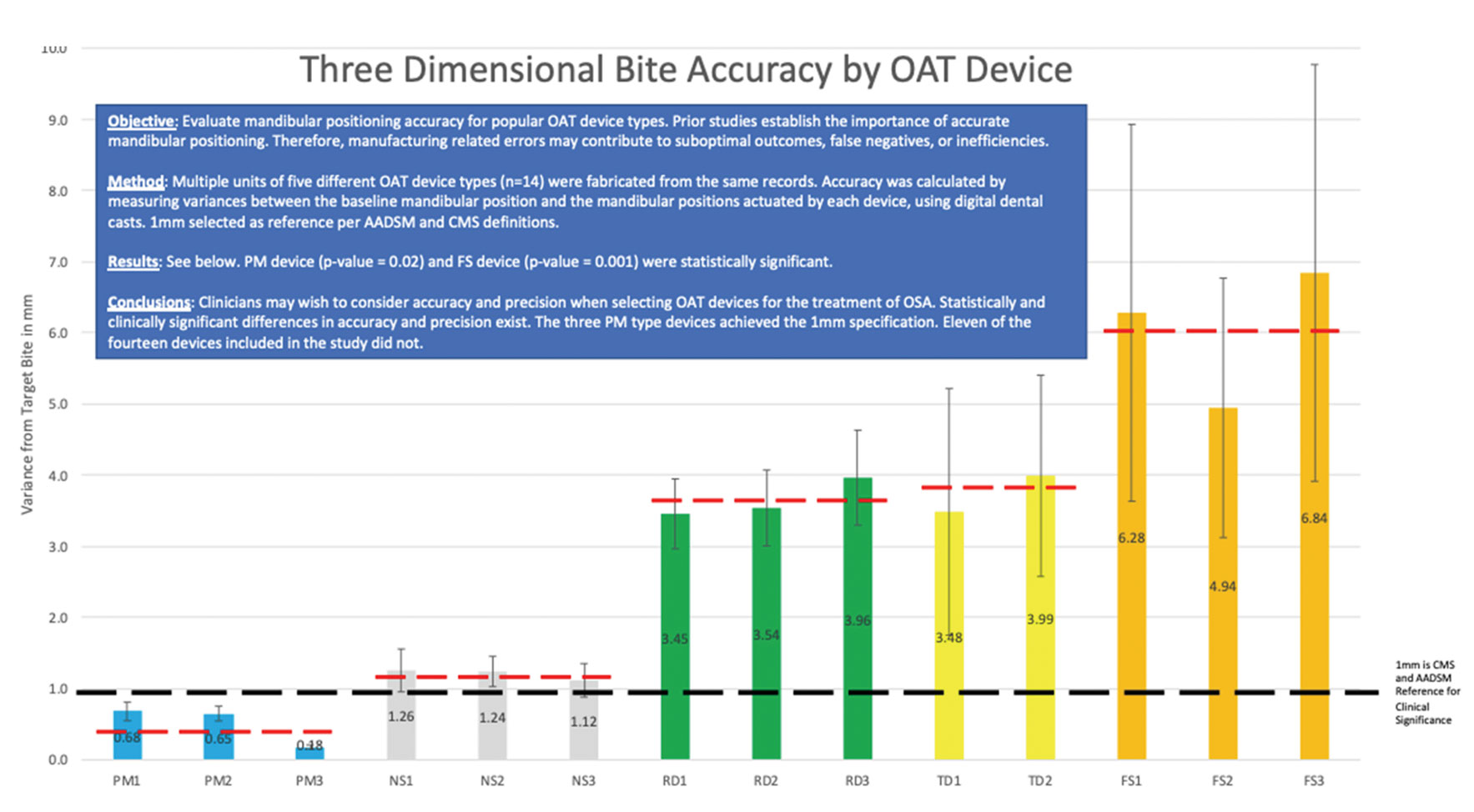
Size
If we want to earn more prescriptions, the OAT device must be comfortable to wear. The primary measures of patient comfort have been overall size, bulk, and preventing irritation and side effects. Multiple evaluations of different manufacturers’ devices made for the same patient have yielded data showing that the ProSomnus precision OAT devices are significantly smaller than comparable predicates. Most recently, a late breaking abstract for the 2020 AADSM (Fig. 2), compared volumes using the Archimedes volume displacement test. The precision OAT devices were 30% smaller than their nearest comparable (nylon strap design) and 66% smaller than the traditional dorsal style predicates. Even the new fulcrum strap OAT device design, a milled device platform with an additional soft liner bonded in, was nearly double the volume.
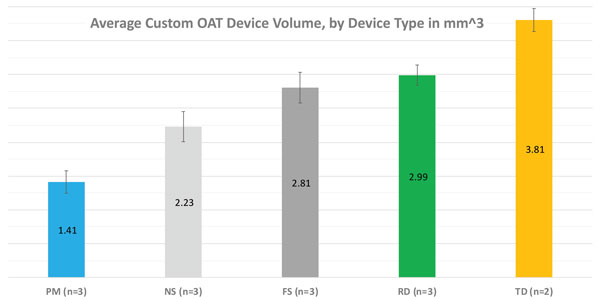

Durability
Simply put, a monolithic material without moving parts or pieces would present as the most durable platform. The ProSomnus [IA] Iterative Advancement Device has no additional materials than the control cured precision milled PMMA. The strength surpasses other comparable device strength and is still smaller. Even the ProSomnus [PH] Precision Herbst-style Device with two Herbst arms added showed superior strength to other predicates. The arms bent at the failure point while preventing catastrophic failure of the entire device.5 Milled precision fit component parts add strength when mixed materials are present.
Biocompatibility
Monolithic control cured materials are the new standard for OAT devices. The density and lack of porosity appear healthier, harbor less bio-gunk, and are easier for the patients to keep clean. The addition of metal Herbst arms or jackscrews always presents an added material compatibility risk. Printed nylon materials are monolithic, but laser melting is not control cured and they are quite porous. The TAP devices utilize a nickel-free alloy on their attachment mechanism but still have the artisanal handmade acrylic. Dual soft liner materials delaminate, stain more, and the porosity harbors far more biogunk.
Easy Delivery
Some devices are more forgiving at initial delivery and would therefore be considered ‘easier to seat.’ This comes with a price. The ‘forgiveness’ may involve additional flexibility that allows for unwanted tooth movement. One of the main disadvantages of liners is that they mask errors in impressions and bites that that you probably want to know about. Similarly, the presence of ball clasps usually indicates a device that is only retentive at those locations. That leaves the remainder of the ‘forgiving’ fit at greater risk. The presence of ball clasps may increase the likelihood of unwanted tooth movement.
Physician preferences towards OAT is largely predicated on how we treat patients and with what generation of OAT devices. Their concerns are Efficacy, Patient Comfort, Side Effects, Compliance, Insurance Coverage, and our Credential Qualifications. Using better devices, with better efficacy and patient comfort, along with fewer side effects will help cross the chasm between PAP and OAT as first line treatments for OSA. Until some new material comes along with the level of precision, strength, and biocompatibility of milled PMMA, we should not compromise ‘easier to seat’ for the characteristics that physicians want, and our patients need.
Dr. Mark Murphy speaks about the “Gold standard of OAT” and discusses oral appliance therapy, COVID-19, and dental sleep medicine in this ZZZ Pack podcast. https://dentalsleeppractice.com/zzz-pack-podcast/mark-murphy-covid-19-dental-sleep-medicine/
- ProSomnus Online Customer Survey, April 2020.
- Vranjes et al, “Assessment of Potential Tooth Movement and Bite Changes With a Hard-Acrylic Sleep Appliance: A 2-Year Clinical Study”; JDSM Vol. 6, No.2 2019.
- Norhem, et al, “Changes in lower incisor irregularity during treatment with oral sleep apnea appliances” Sleep Breath (2017) 21:607–613.
- Uniken Venema J A.M., Stellingsma C, Doff MHJ, Hoekema A. Dental side effects of long-term obstructive sleep apnea therapy: A comparison of three therapeutic modalities. J Dent Sleep Med. 2018;5(2):39-46.
- Internal Third-Party Strength Test, ProSomnus Sleep Technologies (2019).


 Mark T. Murphy, DDS, D.ABDSM, is an American Board of Dental Sleep Medicine Diplomate and has practiced in the Rochester area for over 35 years. He is the Lead Faculty for Clinical Education at ProSomnus Sleep Technologies, Principal of Funktional Sleep, serves on the Guest Faculty at the University of Detroit Mercy School of Dentistry and as a Regular Presenter at the Pankey Institute. He has served on the Boards of Directors of the Pankey Institute, National Association of Dental Laboratories, the Identalloy Council, the Foundation for Dental Laboratory Technology, St. Vincent DePaul’s Dental Center and the Dental Advisor. He lectures internationally on Leadership, Dental Sleep Medicine, TMD, Treatment Planning, and Occlusion.
Mark T. Murphy, DDS, D.ABDSM, is an American Board of Dental Sleep Medicine Diplomate and has practiced in the Rochester area for over 35 years. He is the Lead Faculty for Clinical Education at ProSomnus Sleep Technologies, Principal of Funktional Sleep, serves on the Guest Faculty at the University of Detroit Mercy School of Dentistry and as a Regular Presenter at the Pankey Institute. He has served on the Boards of Directors of the Pankey Institute, National Association of Dental Laboratories, the Identalloy Council, the Foundation for Dental Laboratory Technology, St. Vincent DePaul’s Dental Center and the Dental Advisor. He lectures internationally on Leadership, Dental Sleep Medicine, TMD, Treatment Planning, and Occlusion.