ProSomnus® EVO™ uses advanced materials and optimized design to achieve robustness and comfort in a device that is 10 times more durable than traditional acrylic.
 by Len Liptak, MBA, Mark T. Murphy, DDS, D.ABDSM, and Keith Batcheller, BS, MA
by Len Liptak, MBA, Mark T. Murphy, DDS, D.ABDSM, and Keith Batcheller, BS, MA
Are humans the fastest runners? No. Cheetahs are twice as fast. Do humans have the best eyesight? Nope. Many birds have double the visual acuity. Are humans the best swimmers? Not even close.
So, why are humans exceptional? To paraphrase Steve Jobs, the ability to build and use tools is what makes humans exceptional. Bicycles amplify human locomotion. Computers amplify our ability to calculate. The industrial revolution represents a collection of tools that amplify human labor. Imagining, building, and using tools have exponentially amplified the human existence since the beginning of time.
“The moment mankind first picked up a stone or a branch to use as a tool, they altered irrevocably, the balance between them selves and their environment.”
– James Burke
Tens of millions of adults need “tools” to breathe at night. Without them their airways collapse, depriving their bodies of oxygen, and eventually causing dire medical, economic, and societal consequences. Although current tools like legacy OAT devices and CPAPs are deemed better than placebo, significant opportunity for improvement exists. A study of 150 sleep physicians identified a willingness to quadruple referrals for OAT if the devices, the tools, could be improved to be more comfortable, reliable, easy to use, and consistently effective every night. Sleep physicians, DSM providers, and patients want a tool they can truly trust. Imagine the impact on DSM if that happened.
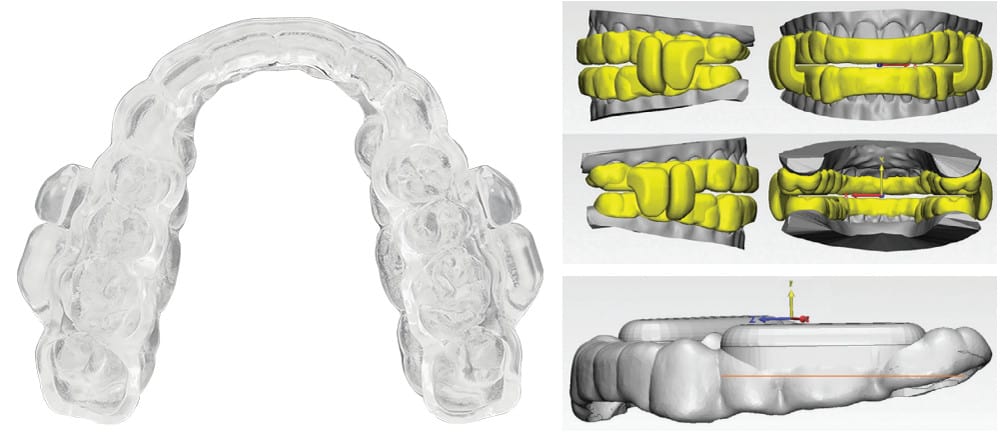
A New Sleep Breathing Tool
ProSomnus® EVO™ is a new FDA Cleared OAT medical device that is engineered to address the challenge for a better sleep breathing tool. Featuring a low profile, anatomical, monolithic, iterative advancement design, EVO is the first OAT device to utilize MG6™ technology. Just as Tesla accelerated the auto industry with the use of advanced materials, Artificial Intelligence, and robotics, ProSomnus is doing this with EVO to create an optimal OAT device. Above all, EVO is designed to earn the trust of DSM providers, sleep physicians, and patients. Let’s take a closer look at this new sleep breathing tool.
Precision Fit and Easy Delivery
One objective for EVO is to make a device that is easy to deliver without compromising precision fit. Liners are a way to achieve easier deliveries. However, liners require significant clinical tradeoffs in the form of suboptimal fit, performance, biocompatibility, bulkiness and repair costs – the very types of quality and cost issues that concern sleep physicians and discourage patients.
ProSomnus EVO uses patented and proprietary innovations to resolve this compromise. EVO is the first OAT device made from a medical grade Class VI rated material. EVO’s advanced material has better modulus, negating the need for liners. This means the material better conforms to dental anatomy and adapts to variances in dental impressions without conceding precision fit, the orthodontic retention of teeth, nor the other tradeoffs associated with liners.
Clinical research indicates that 21% of legacy OATs require problem appointments (Craig et al, 2014). Over 100 EVO cases have been delivered to patients at the time of writing this article. None have required remakes, repairs, or problem appointments.
Robustness and Comfort
Another objective for EVO is to develop a device that is both more robust and more comfortable. There are reasons for DSM providers and OAT manufacturers to chase robustness and comfort. 89% of sleep medicine physicians view poor robustness and 79% view patient discomfort as barriers to more OAT referrals (Granik, 2020). Legacy OAT devices have a high rate of failure, despite efforts to add bulk and reinforcements. One study reported that 13% (41/309) of legacy OAT devices required manufacturer repairs or remakes (Craig et al, 2014).
“Every appliance has pros and cons. I feel like ProSomnus EVO has finally found the best of all worlds. It is small, strong, precise, but overcomes the stiff feeling and offers a flex that patients find more comfortable. I am a happy camper!”
– Erin Elliott, DDS
EVO uses advanced materials and optimized design to achieve robustness and comfort. EVO material is 10 times more durable than traditional acrylic. EVO features a monolithic splint design, with no secondary components like titration screws or straps. In safety testing for the FDA clearance process, EVO exhibited 52% better anterior/posterior torque strength and 17% better lateral torque strength than the predicate device. As expected, predicate devices exhibited catastrophic failures such as fractures, breakage, permanent stretching or plastic deformations, the EVO material flexed and returned to its pre-test shape. Surprisingly, EVO defeated the third-party laboratory destructive testing.
EVO features a true anatomical, low profile, comfortable design made possible by MG6 technologies. Overall, EVO is 2.5 times smaller than the average legacy OAT device. EVO also has an optimized, low profile design: 5.0 times lower profile in the lingual areas, 1.5 times lower profile in the facial areas, and 1.3 times lower profile in the titration mechanism areas. The EVO splint component is uniquely designed based on a mirroring of the actual dental anatomy for each patient to create a familiar, comfortable mouth feel.
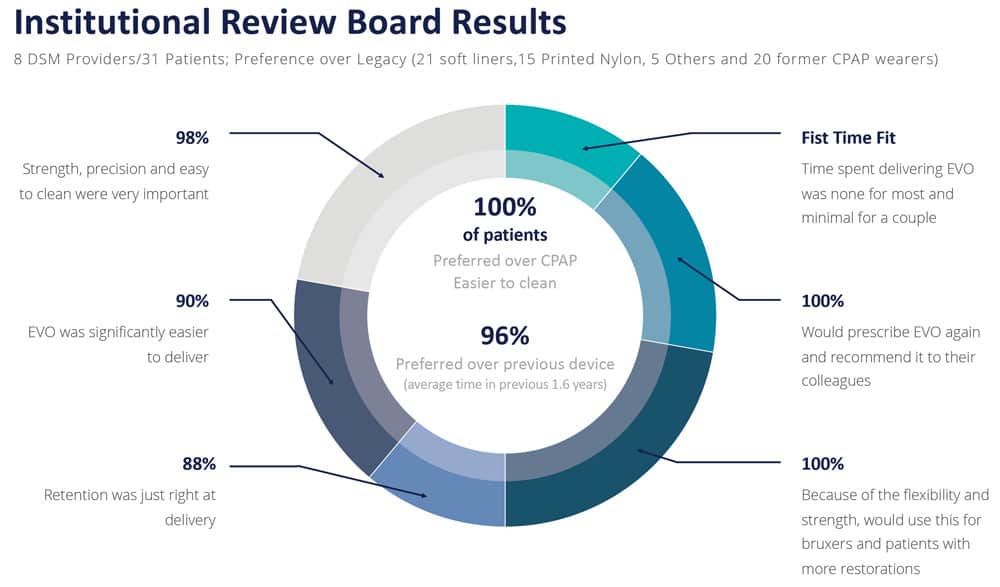
In the IRB study, 100% of patients (21/21) preferred EVO to their CPAPs. 100% (20/20) stated that they would wear EVO more than their CPAPs. 100% (21/21) said EVO would be significantly easier to keep clean than their CPAPs.
93% (26/28) of patients in the IRB study strongly preferred EVO to their legacy OAT devices. 97% (27/28) said it was easy to close their lips together with EVO. 93% (26/28) strongly agreed that EVO was smaller than their previous OAT device. 97% (29/30) strongly agreed that EVO’s anatomical contours had a more natural, smooth feeling.
“Being able to slightly flex the EVO device during insertion allows easier placement
which is ideal for patients who struggle with stretching their oral commis sures. An oral appliance that is flexible and impermeable to external nasties? Are you kidding?”
– Kent Smith, DDS, D.ABDSM, D.ASBA
Biocompatibility and Stain Resistance
Open up an EVO container. How does it smell? When opening a container for a legacy OAT device it usual smells of residual monomers. Even the newer CAD/CAM hybrid devices still smell like chemicals.
Not EVO. EVO material is classified by the United States Pharmacopeia and National Formulary as Class VI. Class VI is the highest grade of material currently available. To achieve Class VI designation, a material must pass a battery of oral, subdermal, and intramuscular toxicity tests.
The material for EVO was selected because it satisfied the clinical performance requirements of leading DSM providers while also being Class VI. The main downside of this material is that it is more expensive. Until recently, ProSomnus did not have the buying power to purchase this material at a reasonable price.
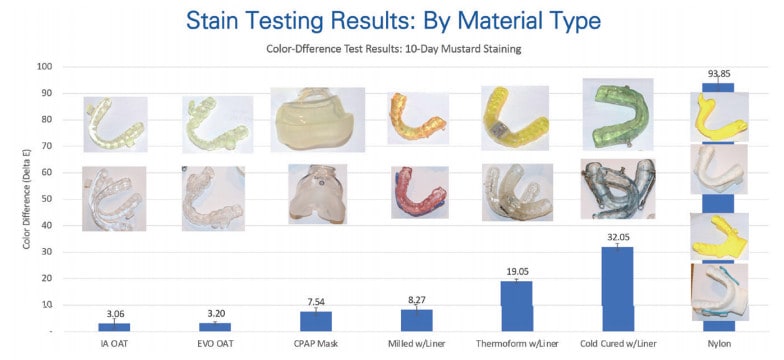
EVO material is highly resistant to staining. Unlike the lower performance materials used in soft liners, acrylic, and nylon devices, EVO material has better modulus without being as porous.
To test stain resistance, an EVO device, a CPAP mask, and a selection of OAT devices were subjected to a 10-day mustard bath test (Fig. 5). A colorimetry score (Delta E) was captured before and after the mustard bath and compared. EVO exhibited virtually no change in color and was on par with the ProSomnus [IA] test device. The CPAP mask was next best, outperforming the traditional acrylic, thermoform, lined CAD/CAM, and nylon material devices.
Precision OAT Evolved
All manufacturing processes have errors. Think about playing golf or taking an impression. Why is one’s golf swing not perfectly the same every time? Why are impressions not perfect every time? It is because variability error exists in the processes, the materials, the environmental factors, and more.
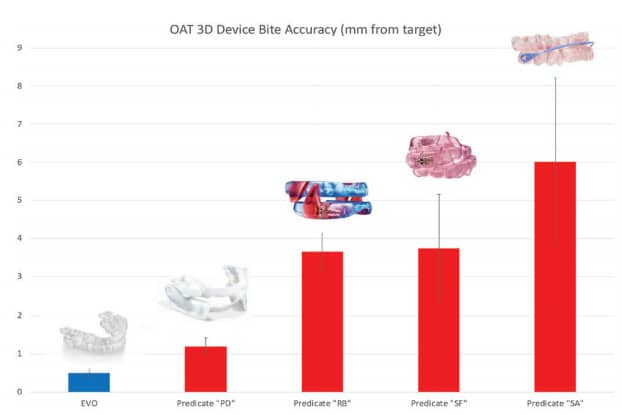
These types of variance errors also exist when transferring a DSM provider’s bite into the design of an OAT device. Some legacy OAT manufacturing processes have over 50 steps – 50 opportunities for variability! An investigation of bite transfer precision found that the average legacy OAT device has 3.7mm, ranging from 1.2mm to 6.0mm, of global variance between the bite provided and the setup of the device (Hu, 2020). This is significant when considering that the mean protrusive range of the mandible is 8.0mm.
ProSomnus devices reproduce the bite accuracy within 0.32mm (Fig. 6). This is largely due to MG6 technologies, including Artificial Intelligence and manufacturing robots, that reduce process steps and variability. ProSomnus also digitally or physically mounts and articulates every case for quality control.
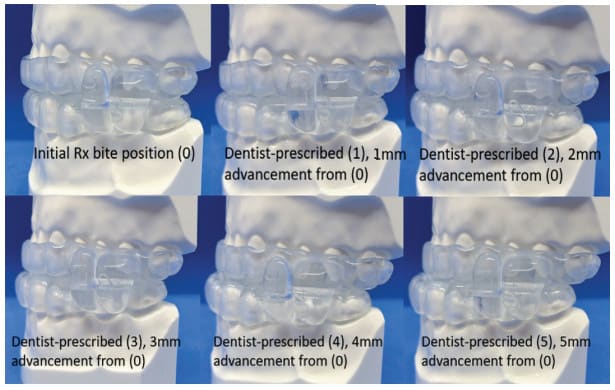
To further add value and reduce costs for the DSM practice, EVO comes standard with five arches. This enables 5.0mm of advancement and creates a built-in backup device if a patient loses an arch or a device (Fig. 7). The backup allows the patient to remain in some level of therapy until a replacement can be made.
“With their sleek design and stain resistance, ProSomnus devices already sell themselves. To now also get material flexibility with the MG6 technology which increases patient comfort and reduces chair-time is just icing on the cake!”
– Srujal H. Shah, DDS, D.ABDSM
Overall Impressions
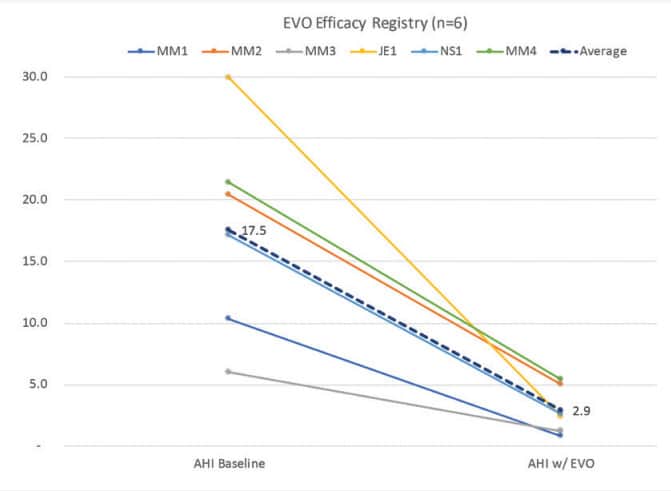
Preliminary efficacy results with EVO show a 83% improvement in AHI, from a baseline of 17.5 to 2.9 (Fig. 8). Some DSM providers will care about certain OAT features and advantages more than others. Some will care about lip competency more than tongue space. Some will care about precision 3D bite transfer more than device bulkiness. Others will have the opposite preferences. Preferences are an important part of ensuring that the OAT device best fits the needs of the patient and the treatment plan for the DSM provider.
What are the overall impressions of EVO? 100% (31/31) would prescribe EVO again. 97% (30/31) would feel very confident prescribing EVO for bruxers. Another 94% (29/31) would feel very confident prescribing EVO for patients with crowns, bridges, or veneers. 97% (30/31) stated that they strongly favor using EVO to treat a wider range of patient types. And most importantly, 100% (31/31) stated that they would recommend EVO to a colleague.
ProSomnus’s mission is to improve the lives of millions by designing better tools for DSM providers – tools like the EVO that help people breathe at night. We are wholly committed to providing tools that DSM providers find easy to use, patients love to wear, and physicians trust when prescribing. Join us on this mission.
ProSomnus warmly thanks the dozens of DSM leaders and team members who participated in market research programs, prototype evaluations, and the IRB, alpha and beta tests that were integral to the design and development of EVO.
Read more about ProSomnus® EVO™ receiving its FDA clearance here: https://dentalsleeppractice.com/industry-news/prosomnus-evotm-oral-appliance-therapy-device-receives-fda-clearance-for-the-treatment-of-obstructive-sleep-apnea-and-snoring/
 Mark T. Murphy, DDS, D.ABDSM, is an American Board of Dental Sleep Medicine Diplomate and has practiced in the Rochester area for over 35 years. He is the Lead Faculty for Clinical Education at ProSomnus Sleep Technologies, Principal of Funktional Sleep, serves on the Guest Faculty at the University of Detroit Mercy School of Dentistry and as a Regular Presenter at the Pankey Institute. He has served on the Boards of Directors of the Pankey Institute, National Association of Dental Laboratories, the Identalloy Council, the Foundation for Dental Laboratory Technology, St. Vincent DePaul’s Dental Center and the Dental Advisor. He lectures internationally on Leadership, Dental Sleep Medicine, TMD, Treatment Planning, and Occlusion.
Mark T. Murphy, DDS, D.ABDSM, is an American Board of Dental Sleep Medicine Diplomate and has practiced in the Rochester area for over 35 years. He is the Lead Faculty for Clinical Education at ProSomnus Sleep Technologies, Principal of Funktional Sleep, serves on the Guest Faculty at the University of Detroit Mercy School of Dentistry and as a Regular Presenter at the Pankey Institute. He has served on the Boards of Directors of the Pankey Institute, National Association of Dental Laboratories, the Identalloy Council, the Foundation for Dental Laboratory Technology, St. Vincent DePaul’s Dental Center and the Dental Advisor. He lectures internationally on Leadership, Dental Sleep Medicine, TMD, Treatment Planning, and Occlusion. Len Liptak, MBA, is the CEO of ProSomnus® Sleep Technologies. An award-winning executive with expertise growing and operating innovation-oriented businesses, Len is a founding member of ProSomnus, and co-inventor of the company’s flagship product. Len also serves on the company’s Board of Directors. Len earned an MBA from the University of Minnesota’s Carlson School of Management and a BA from Brown University. A lifelong learner, Len has completed executive education programs at John’s Hopkins, and is a member of the Young President’s Organization (YPO).
Len Liptak, MBA, is the CEO of ProSomnus® Sleep Technologies. An award-winning executive with expertise growing and operating innovation-oriented businesses, Len is a founding member of ProSomnus, and co-inventor of the company’s flagship product. Len also serves on the company’s Board of Directors. Len earned an MBA from the University of Minnesota’s Carlson School of Management and a BA from Brown University. A lifelong learner, Len has completed executive education programs at John’s Hopkins, and is a member of the Young President’s Organization (YPO). Keith Batcheller is the Vice President of Product Marketing for ProSomnus Sleep Technologies. Prior to starting with ProSomnus, Keith was an Orthodontic Business Development Specialist/Consultant for companies in Asia, Europe and North America. Keith has 21 years in the dental/orthodontic space and worked for companies like 3M, Burkhart Dental, Henry Schein Orthodontics and ClearCorrect. Keith was the recipient of the Golden Step Award by 3M for the successful innovation and commercialization of the SmartClip™ Self-Ligating Appliance System and has helped develop and commercialize many of the orthodontic products on the market today. Keith holds a Bachelor of Science degree in Microbiology from the University of Washington and a Master’s degree from Multnomah University in Leadership and Development.
Keith Batcheller is the Vice President of Product Marketing for ProSomnus Sleep Technologies. Prior to starting with ProSomnus, Keith was an Orthodontic Business Development Specialist/Consultant for companies in Asia, Europe and North America. Keith has 21 years in the dental/orthodontic space and worked for companies like 3M, Burkhart Dental, Henry Schein Orthodontics and ClearCorrect. Keith was the recipient of the Golden Step Award by 3M for the successful innovation and commercialization of the SmartClip™ Self-Ligating Appliance System and has helped develop and commercialize many of the orthodontic products on the market today. Keith holds a Bachelor of Science degree in Microbiology from the University of Washington and a Master’s degree from Multnomah University in Leadership and Development.