 Why is home sleep testing an important tool for sleep apnea treatment providers?
Why is home sleep testing an important tool for sleep apnea treatment providers?
Perhaps the greatest challenge presented to the sleep apnea dentist is the fact that calibrating the oral appliance on an awake patient leaves you with, um,…a well-adjusted awake patient. The patient’s sleep apnea occurs unobserved at night and often one night is not exactly like another night. How then can the clinical team avoid over- and under-titrations (recognized as the major cause of treatment noncompliance)? Or in cases where the clinical team has decided that from a dental perspective, further appliance titration may compromise dentition or raise joint concerns, can they select a combination therapy with CPAP, positional therapy or weight loss to support a dental sleep apnea treatment plan?
For years there was no option beyond full in lab polysomnogram (PSG). Even with all of its flaws it would be hard to argue that PSG isn’t the best way to examine sleep efficiency and quantitively evaluate sleep treatments (PAP is perfect for this model). The challenge for dentists is that the cost of attended studies is just too high to use PSG for titration, leaving home sleep testing as the only option. In the United States, insurance companies have begun to resist PSG in favor of home diagnostic for simple sleep apnea (their term, not mine). The great news is that these sleep diagnostic devices are giving dentists the ability to calibrate their appliances with a degree of accuracy that is similar to a CPAP titrated in the sleep lab.
I feel that when comparing Continuous Positive Airway Pressure (CPAP) therapy with Oral Appliance Therapy (OAT) there are a few major differences generally centered on the initial calibration and ongoing titration. CPAP is titrated in lab with a sleep technician observing the sleeping patient while adjusting the CPAP pressure (often this is the only time the patient’s pressure will be calibrated). Oral Appliances, on the other hand, are titrated in the dental office using subjective patient or bed partner reports of snoring and sleepiness. A further complication is that as patients habituate to the appliance, muscles and ligaments relax and lengthen over the first few months of appliance wear. The patient may be 100% compliant with the appliance however the adjustment may no longer be adequate to maintain a patent airway. The dental relationship is such that the patient will receive an annual or biannual follow-up in order to manage their therapy. Home testing is an important part of this ongoing management of the patient’s sleep apnea.
Clearly, using a home sleep diagnostic device gives the clinical team the opportunity to monitor, evaluate and adjust therapy to suit each individual’s needs.
So how to select an HST device that will provide enough clinical value while at the same time be simple enough for the patient to use unattended in their home?
What choices are available for sleep testing?
 HST devices fall into two basic categories: Screeners and Home Sleep Diagnostic devices.
HST devices fall into two basic categories: Screeners and Home Sleep Diagnostic devices.
A screener (type IV: 1 or 2 channels usually using oximetry as 1 of the parameters) is designed to provide enough information to identify that the patient has sleep disordered breathing but without enough information to establish a treatment pathway. Data driven discussions of sleep quality initiated by patients using portable and wearable sleep tracking devices is a very new trend in patient education.
It goes without saying that the 1994 guidelines couldn’t predict this recent trend. The wearable sleep tracker device revolution has really started to get into the Type IV sleep screener arena with products that are steadily improving in accuracy and usability. The Apple Watch, for example, has a sophisticated heart rate monitor that is capable of acting as a pulse oximeter. This feature is turned off in the device so it is a product for the future. It remains to be seen if the Apple Watch will qualify as a level IV device. Based on the current guidelines and the computing power of the Apple Watch, the product may eventually have the feature set to reach Level III Home Sleep Diagnostic Device capability.
Mattress manufacturers have started pursuing sleep quality data as a way of encouraging mattress choices based on style data. The Beddit 3 Sleep Tracker for example is a thin flexible sensor that lies across the mattress reading heart rate, breathing, temperature, humidity and even snoring. The folks at Sleep Number have released a product, Sleep IQ, that tracks sleep quality and connects to your favorite health and wellness apps. Is it possible that diagnostic mattresses will test the line between screener and diagnostic device?
 Today wearable devices like Jawbone, Fitbit or bedside monitors like the S+ from ResMed are designed to provide data about sleep efficiency and sleep quality. In general, these devices provide excellent sleep quality data. They cannot provide data that can be used to determine appropriate treatment pathways, and reliability and accuracy have been concerns. Wearable trackers may record the subject to be asleep when they are simply sitting and watching TV. Clearly, there is still some work to do in this area.
Today wearable devices like Jawbone, Fitbit or bedside monitors like the S+ from ResMed are designed to provide data about sleep efficiency and sleep quality. In general, these devices provide excellent sleep quality data. They cannot provide data that can be used to determine appropriate treatment pathways, and reliability and accuracy have been concerns. Wearable trackers may record the subject to be asleep when they are simply sitting and watching TV. Clearly, there is still some work to do in this area.
The big question when working with patients who use fitness trackers is how to use the reams of information that the patient presents with. To date there are no standards in the “wellness” field, which is where the trackers are rated by the FDA.
Many patients realize that the sleep tracker is not a medical grade device however when compared to the fee for a sleep study some patients are willing to compromise on accuracy in favor of low cost. The clinician, however, cannot work with the data regardless of the amount of detail in the Excel spreadsheet. There just isn’t a lot of medical information to be gleaned from number of steps in a day or how restless the patient was over the last week. This presents a relationship dilemma requiring more skills and often more clinic time to merge the patient’s expectations with clinical reality.
The key to success with level IV devices rests with moving the patient into a home sleep diagnostic device so that a therapy can be prescribed and delivered in a way that can be objectively monitored, titrated and managed.
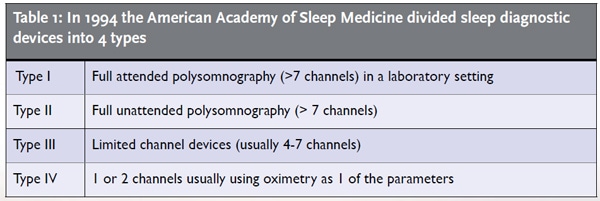
The Home Sleep Diagnostic Devices category (Type III limited channel devices 4-7 channels) is much more crowded and the decisions are complicated by insurance reimbursement and other factors. The guidelines used today were established by a task force in 2007 by the American Academy of Sleep Medicine (AASM). The guidelines for portable monitoring state that at minimum a device must record airflow, effort and oximetry, biosensors conventionally used for in lab PSG. This leaves a tremendous range of equipment capability, from units with few measured parameters like the ARES from SleepMed or the WatchPat from Itamar, to devices with 18 channels or more like the Nox T3 from CareFusion or the MediByte from Braebon. There are many more.
The task force went on to determine that whichever device is selected, the raw data from the device should be output for scoring by a sleep technician and interpretation by a board-certified sleep physician. Devices at one end of the spectrum cannot share a common description with devices on the other end of the spectrum, yet most of the devices on the market meet these broad guidelines.
So what are the key features that may make the difference to the dental clinician?
In my opinion these criteria should be taken into account before committing to a home testing device. There are a number of opinions in the works that may affect how we all will work with these devices. Below is a top 5 list of things to look for (barring a change in guidelines)
- Does the device meet or exceed AASM level III definition. This is a requirement for reimbursement. Insurance companies tend to use national regulatory bodies to establish standards. If the standards change, so too might the value of your equipment. I understand that there are new standards in the works and that some States have established their own standards and rules. Research your region or at minimum pick a national standard and follow that one.
- Cost per test and cost per patient are terms that are used interchangeably? High per-test disposable costs may be acceptable in a diagnostic situation but in a titration protocol with a challenging case, several tests may be required. In that case, more reusable materials help keep costs down. Cost and complexity are the two biggest impediments to home testing in the dental office. Rule of thumb is the more you test, the better your outcomes.
- Has the device been validated against PSG?
- PSG is the standard in sleep testing make sure the device you use has been thoroughly tested against the gold standard.
- I believe the key phrase in the AASM guidelines is “the airflow, effort and oximetric biosensors conventionally used for in-laboratory PSG should be used in PM.” (PM is Portable Monitoring or Home Diagnostic Testing). It the device you select uses methods that are not in use in sleep labs you should find out why.
- Guidelines suggest that a device should be able to use thermistor or pressure transducer for apnea detection and hypopnea detection respectively
- Respiratory effort should be identified with calibrated or uncalibrated inductance plethysmography
iii. Blood Oxygen should be detected using pulse oximetry with signal averaging time and accommodation for motion artifact.
Plethysmography for arousal detection using Pulse Wave Analysis is new and should be considered
- Snoring recorded using audio, not from the nasal cannula, per recent research
- Alternate channels should be avail-
able to collect data like Bruxism, ECG, EMG, EOG - Scoring according to the AASM Manual for Scoring of Sleep and Associated Events is also a requirement, same as the PSG lab.
- Body position is a critical parameter for dental patients and can mean the difference between treated and untreated for patients with compromised dentition or TMJ issues
- Perhaps the most important aspect of any home sleep device is the following: “Can the patient attach the device and initiate a study in their home without problems?”
If the patient does not return in the morning with a good test or the device was hot, loud, distracting or impossible to turn on, the study will not yield what is required. Retests cost money, especially if the per test price is high or if more office time is required to train the patient. Sleep labs who dispense HST often have 24-hour troubleshooting support – if you want to provide that from your dental office, choose a device that is least likely to generate a call. Patient satisfaction will suffer and, perhaps worst of all, staff confidence will take a hit.
The optimum home sleep testing device to buy for your practice is the one that is out every night on patients, indispensably part of your sleep therapy.
